Novel expression vectors and uses thereof
A technology of expressing vectors and vectors, applied in the field of new vectors, can solve the problems of reducing efficacy, human genome danger, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0263] Cloning and analysis of expression properties of plasmids in series 1 and NNV
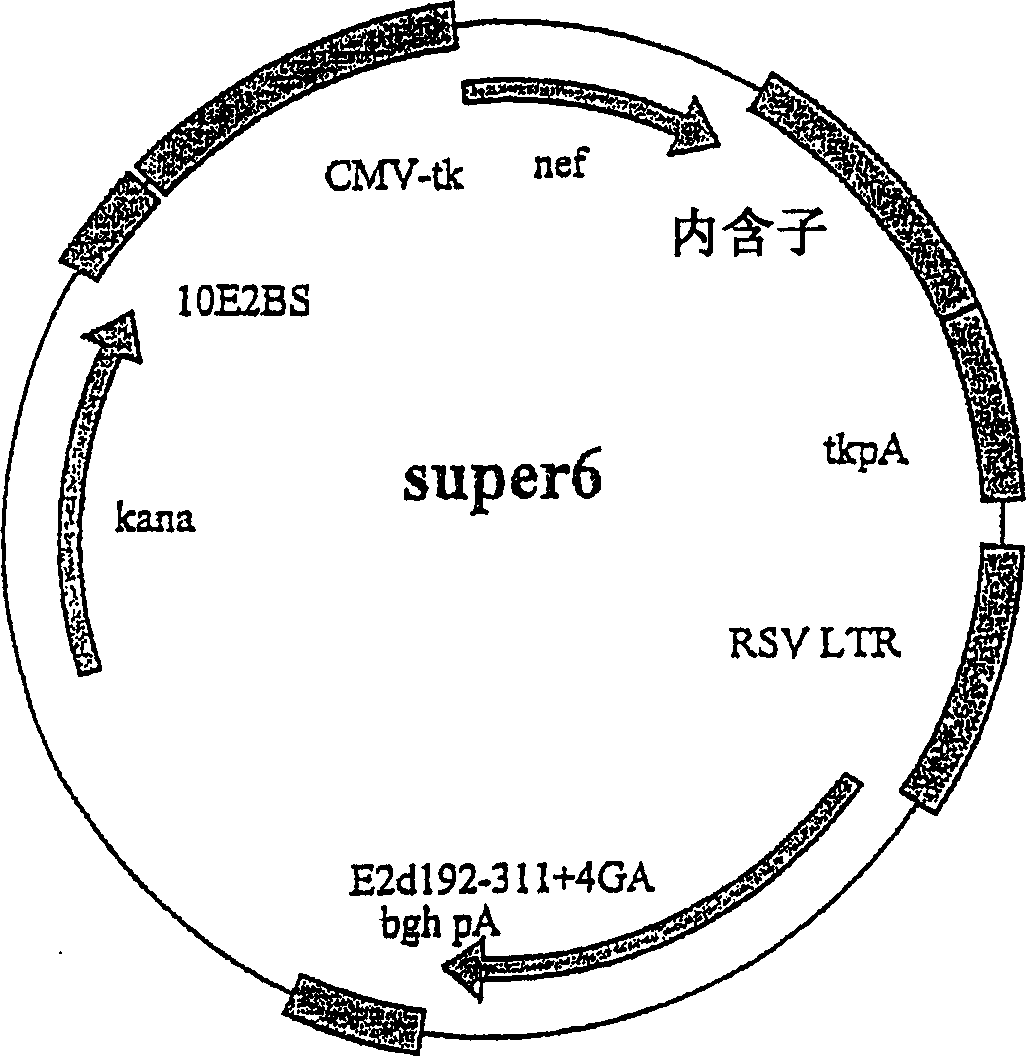
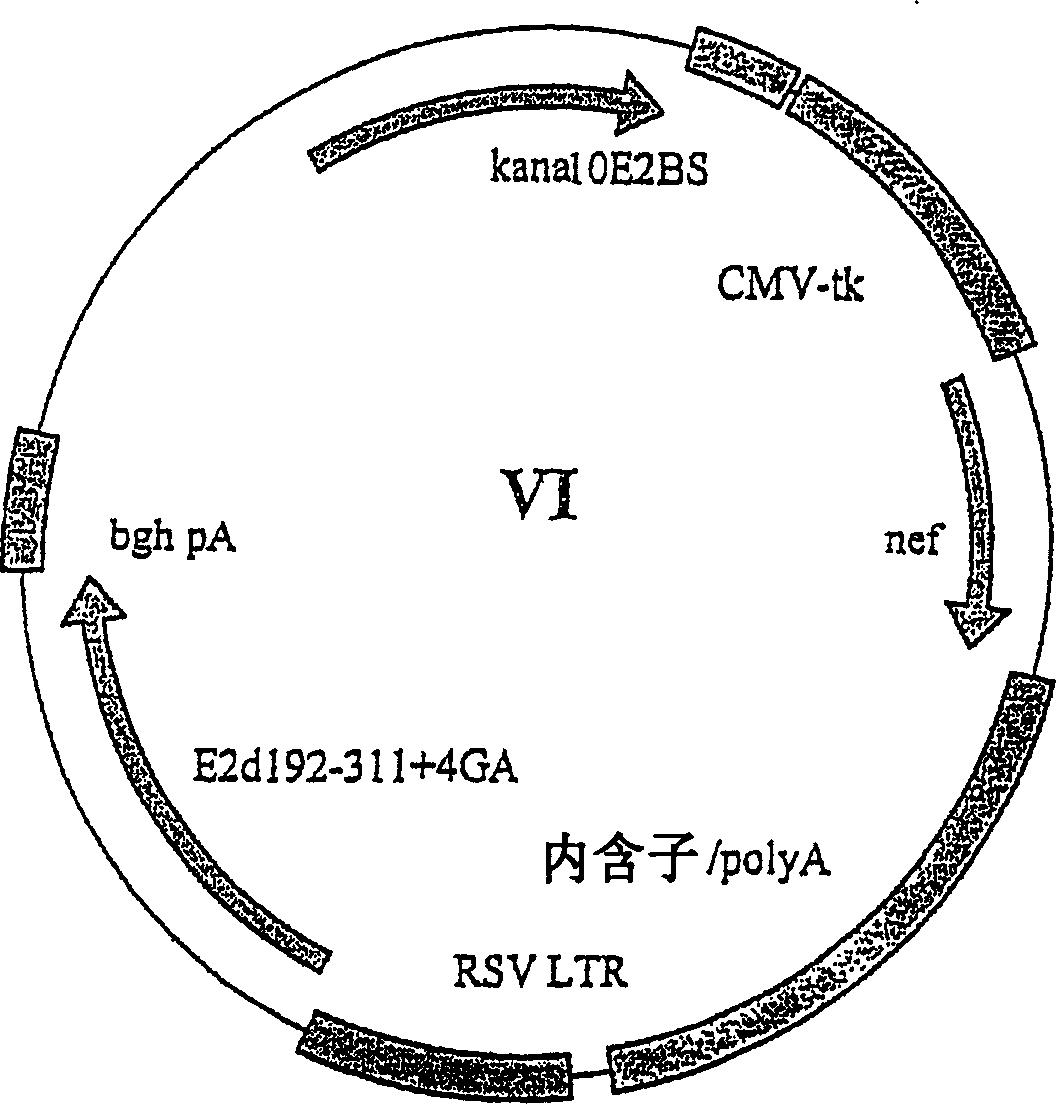
[0264] Further modifications were made in these vectors in order to increase the copy number of the vectors super6 and super6wt in E. coli. The Tn903 kanamycin resistance gene, pMB1 replicon and ten E2 binding sites were removed by HindIII / Nhe1 digestion and then replaced with the HindIII / Nhe1 fragment of the retroviral vector pBabe Neo [Morgenstern, J.P. and Land, H. ., Nucteic Acids Research 18(1990) 3587-3596]. This fragment contains a modified pMB1 replicon and the Tn5 kanamycin resistance gene, allowing loose high copy number replication of the plasmid in bacteria. The new plasmids were named Product 1 (Figure 5) and Product 1wt, respectively. Reinsertion of the ten E2 binding sites back into the blunt Nhe1 site upstream of the CMV promoter in product 1 resulted in unsuccessful results in the generation of the new vector NNV, respectively, with only two binding sites integrated into t...
example 3
[0269] Analysis of expression characteristics of NNV-2wt
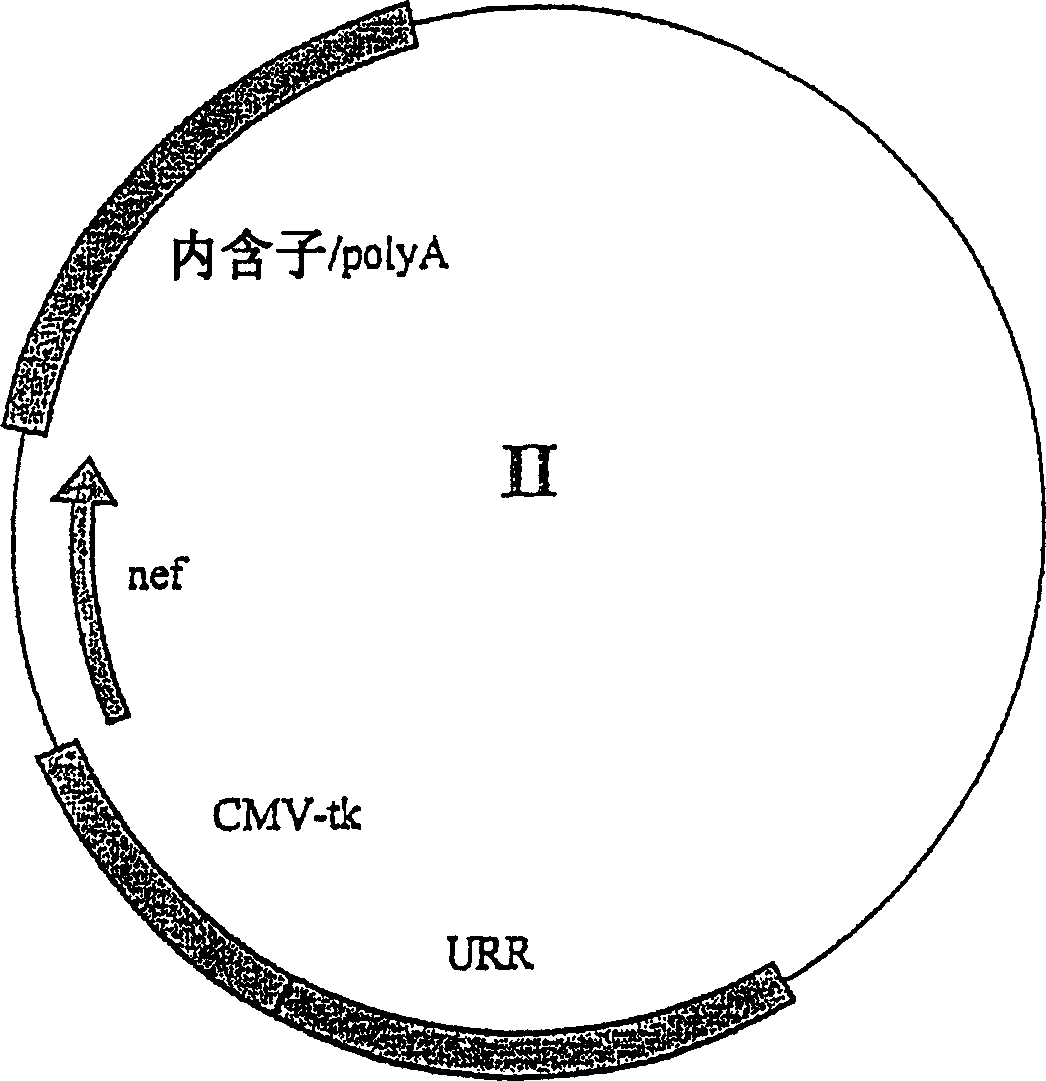
[0270] In order to analyze the expression characteristics of NNV-2wt, four different cell lines, namely Jurkat (human T cell lymphoblastoid cells), P815 (mouse mast cell tumor cells), CHO (Chinese hamster ovary cells) lines and RD (human embryonic striated muscle sarcoma cells), were transfected by electroporation, and their expression of Nef and E2 was analyzed. To demonstrate the transcriptional activation and maintenance properties mediated by E2 proteins and E2 oligomerization binding sites, the product 1wt lacking E2 binding sites (Figure 5) was used as a control. An additional control plasmid is the plasmid NNV-2wtFS, which differs from NNV-2wt by an introduced frameshift in the E2 coding sequence, so it does not express functional E2 protein.
[0271] Each cell line was essentially transfected by electroporation as described in Example 1 with different amounts of vector DNA. Time points were determined approxi...
example 7
[0299] Analysis of NNV-2wt replication in the presence of human papillomavirus replication factors
[0300]Papillomavirus proteins have previously been shown to initiate replication of heterologous ori-containing plasmids from many other human and animal papillomaviruses [Chiang, C.M. et al., Proc Natl Acad Sci USA 89 (1992) 5799-5803]. Although NNV-2wt does not contain a complete origin of replication, it was detected how replication is initiated in the presence of HPV type 1 E1 and E2 proteins. CHO cells were treated with 1 mg vector NNV-2wt, NNV-2wtFS or product 1 alone or with 4.5 μg HPV-11 E1 expression vector pMT / E1 HPV11 or with the same amount of pMT / E1 HPV11 and 4.5 μg HPV-11 E2 protein expression vector pMT / E2 HPV11 were transfected together, as shown in the upper part of Figure 15. Transfection was performed essentially as described in Example 1. El and E2 expression vectors have been described previously (Chiang, C.M. et al., supra). An equimolar amount of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com