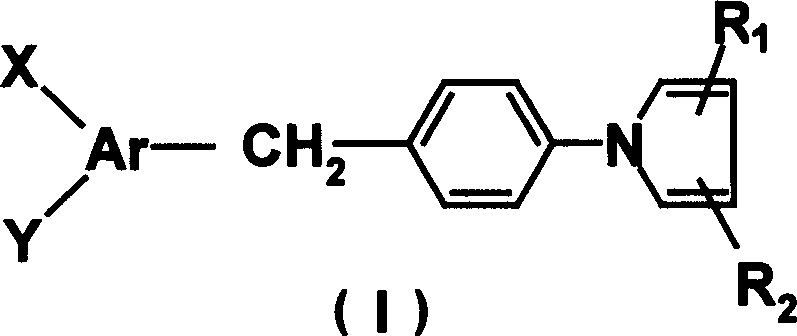
N-aryl substituted-1H-pyrrole derivative possessing cardiovascular activity
A technology of heteroaryl and alkyl, applied in the field of N-aryl-substituted-1H-pyrrole derivatives, can solve the problems of high price and many reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
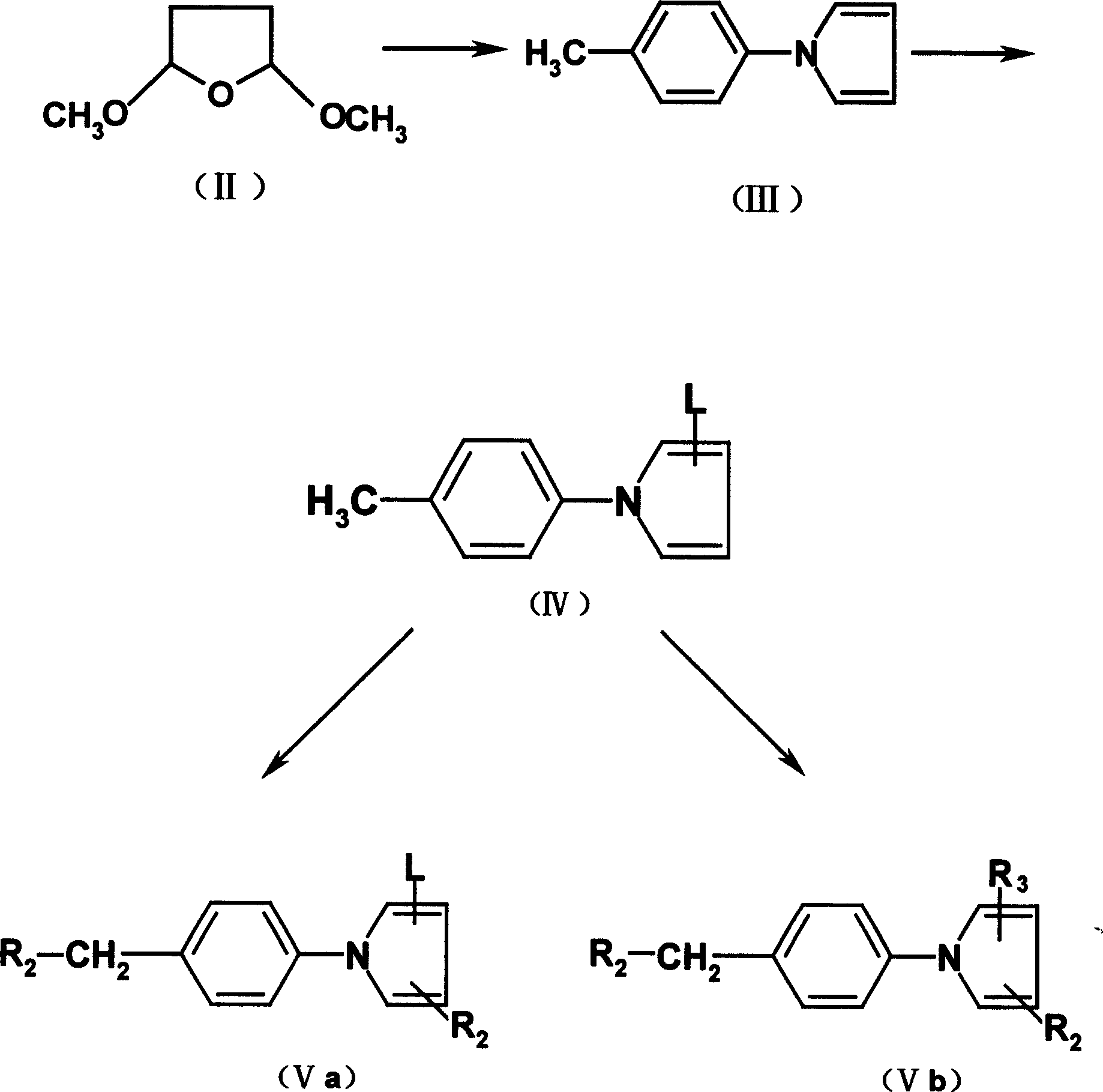
Embodiment 1
[0063] 1-(4-Methylphenyl)-1H-pyrrole
[0064] Dissolve 2,5-dimethoxy-tetrahydrofuran (25g, 0.19mol) and p-toluidine (20.6g, 0.19mol) in 80ml of ice HAc, heat to reflux for 3h, concentrate, and steam distill the residue to obtain 23g (76%) of the title compound as white crystals, mp.80-82°C.
Embodiment 2
[0066] 1-(4-Methylphenyl)-2-formyl-1H-pyrrole
[0067] Under anhydrous conditions, cool DMF (20g, 0.28mol) in an ice-salt bath, slowly add POCl dropwise 3 (43g, 0.28mol), after dropping, it was raised to room temperature, stirred for 20min, then 100ml of DMF solution of 1-(4-methylphenyl)-1H-pyrrole (22g, 0.14mol) was added dropwise thereto, and after dropping, React at 100-120°C for 3h. Cool, pour into ice, adjust PH=9 with 10% NaOH, extract with ethyl acetate 60ml×3, anhydrous Na 2 SO 4 Dry and concentrate to obtain a red oily substance, 22 g (81%) of the title compound, which is directly used in the next reaction.
Embodiment 3
[0069] 1-(4-Methylphenyl)-2-oximino-1H-pyrrole
[0070] The above red oil (15g, 0.08mol) was dissolved in methanol 110ml, heated to reflux, and dissolved in hydroxylamine hydrochloride (5.6, 0.08mol) and Na 2 CO 3 (8.5, 0.08mol) aqueous solution 55ml, dropwise, reflux for 5h, filter with suction, evaporate methanol, the residue is extracted with ethyl acetate 50ml×3, anhydrous Na 2 SO 4 Dry and concentrate to give a yellow oil as the title compound 14g (90%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com