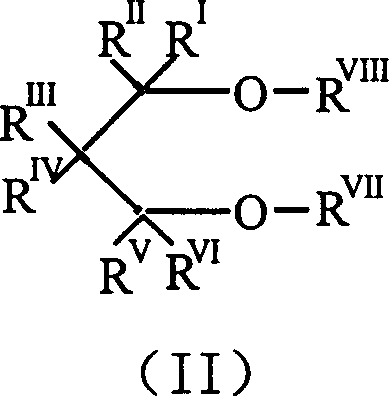
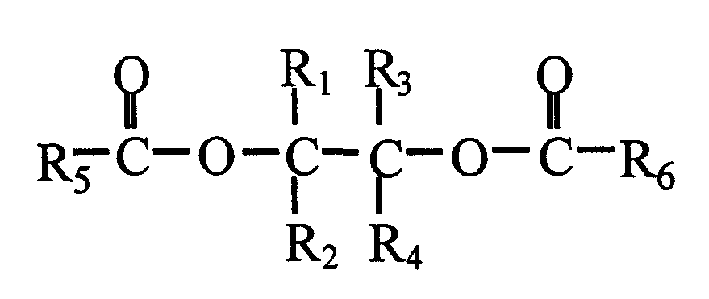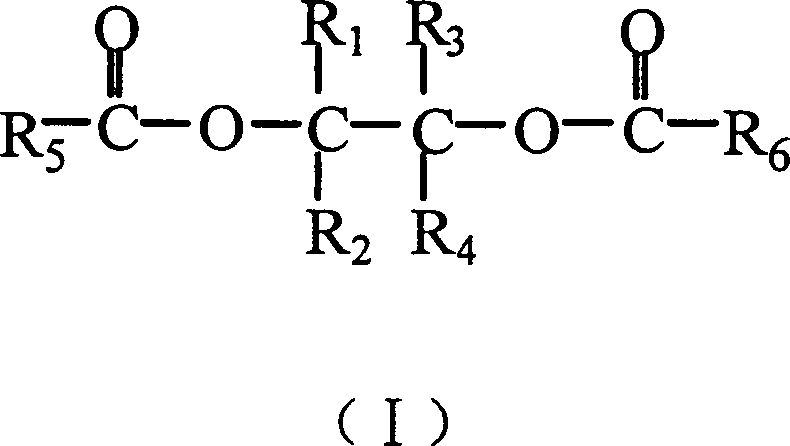Catalyst component for olefin polymerization reaction and catalyst
A technology for olefin polymerization and catalysts, which is applied in the field of catalyst components and catalysts for olefin polymerization reactions, and can solve the problems of narrow molecular weight distribution of polymers, low catalyst activity and orientation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of 1,2-Butanediol Benzoate
[0054] 1,2-Butanediol (2.5g), benzoyl chloride (7.8g), pyridine (8.8g) and tetrahydrofuran (70ml) were added to the reactor, mixed, and heated to reflux for 4 hours. Then it was lowered to room temperature, and water was added to the reaction system until the inorganic phase was transparent. The organic phase was separated, and the inorganic phase was extracted with ether and combined with the organic phase. After washing the organic phase with water, drying the organic phase with anhydrous sodium sulfate, and concentrating, the product was isolated to obtain 3.95 g of product. 1 H-NMR (TMS, CDCl 3 , ppm): δ 1.0-1.1 (3H), 1.7-1.9 (2H), 4.4-4.6 (2H), 5.4-5.5 (1H) and 7.4-8.2 (10H).
Embodiment 2
[0056] Synthesis of 2,3-Butanediol Benzoate
[0057] The synthesis method is the same as that of Preparation Example 1 to obtain 4.4 g of the product. 1 H-NMR (TMS, CDCl 3 , ppm): 1.4-1.6 (6H), 5.3-5.5 (2H) and 7.4-8.2 (10H).
Embodiment 3
[0059] Synthesis of Catechol Dibenzoate
[0060] 50ml of tetrahydrofuran was added to 5.5g of catechol, and 12.1ml of pyridine was added under stirring. After stirring well, slowly add 14.5ml of benzoyl chloride, stir at room temperature for 1h, then heat to reflux for 4h. Add 70ml of water to dissolve the formed salt, extract with toluene, separate the organic phase, wash twice with saturated brine, and dry over anhydrous sodium sulfate. Removal of the solvent gave a white solid. Recrystallize with ethyl acetate to obtain white crystals of catechol dibenzoate with a yield of 94%. m.p.75~77℃. 1 NMR (CDCl 3 ) δ (ppm): 7.35 to 7.54 (m, 10H, aromatic ring hydrogen), 8.05 to 8.12 (m, 4H, aromatic ring hydrogen).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com