Formulations for treatment of mucosal associated conditions with an immune response modifier
A technology of immune response and regulator, applied in the direction of antiviral agents, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of obstruction, poor systemic transmission, poor permeability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0272] Example 1 Safety, pharmacokinetics (PK) and Pharmacodynamic (PD) assessment
[0273] method
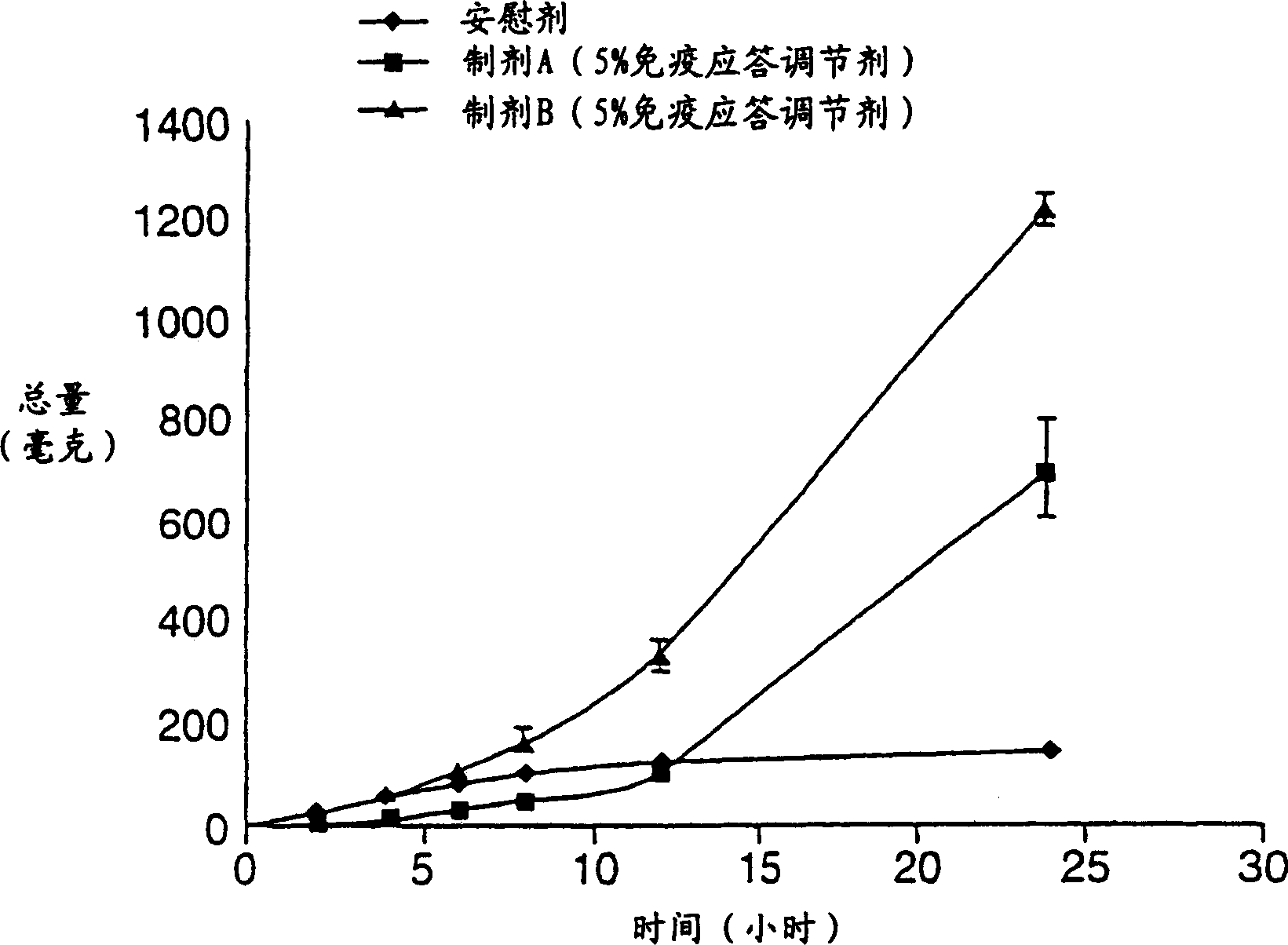
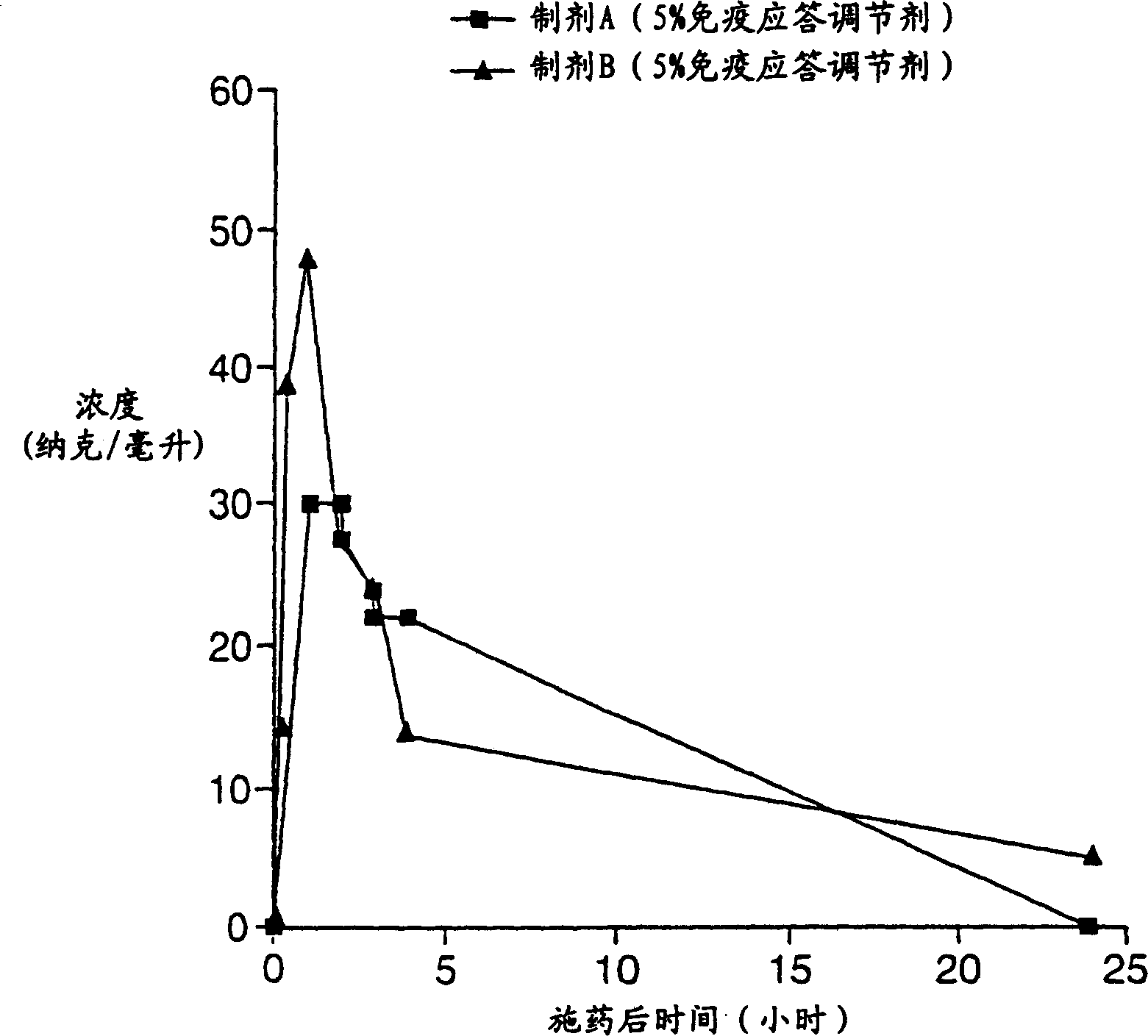
[0274] This was a single-dose, randomized, double-blind dose-escalation study (controlled with placebo) evaluating 5 doses of imiquimod. A cream formulation of 50, 100, 150, 200 and 250 mg of imiquimod was applied to the cervix for 8 hours. The ingredients of the imiquimod cream (Formulation A) used in this study are listed in Table I below. Each dose group consisted of 8 subjects (6 active drug, 2 placebo), two subjects were treated as dose leaders and the remaining 6 were treated following dose leader acceptable response. Safety was assessed by adverse events (AEs), laboratory tests and, if required, colposcopy with a cervical photographic recorder pre-dose, 24 hours post-dose and 48 hours post-dose. Systemic exposure (PK) was determined by measuring imiquimod and its metabolites in 48 hours post-dose, by cytokine: TNF-α ( TNF-α), interferon-α (IFN-α), interleukin-1 ...
Embodiment 2
[0279] Example 2 Preparation of Pharmaceutical Formulation B
[0280] This example describes a novel formulation for vaginal application which is a stable formulation with high viscosity and good shelf life, passing the EP Preservative Effectiveness Test (PET) criteria. The w / w% of the components of this formulation (Formulation B) are listed in Table 2 below.
[0281] Imiquimod and Span85 were dissolved in isostearic acid. Pluronic F68, EDTA, Carbopol 974P, Propylene Glycol, Sorbic Acid and Methylparaben are dissolved in water. After emulsification to form an oil-in-water emulsion, sodium hydroxide is added to reach a pH of about 5.2. The pH range of the formulation is about 4.8-6.0.
[0282] Table 2
[0283] components
Embodiment 3
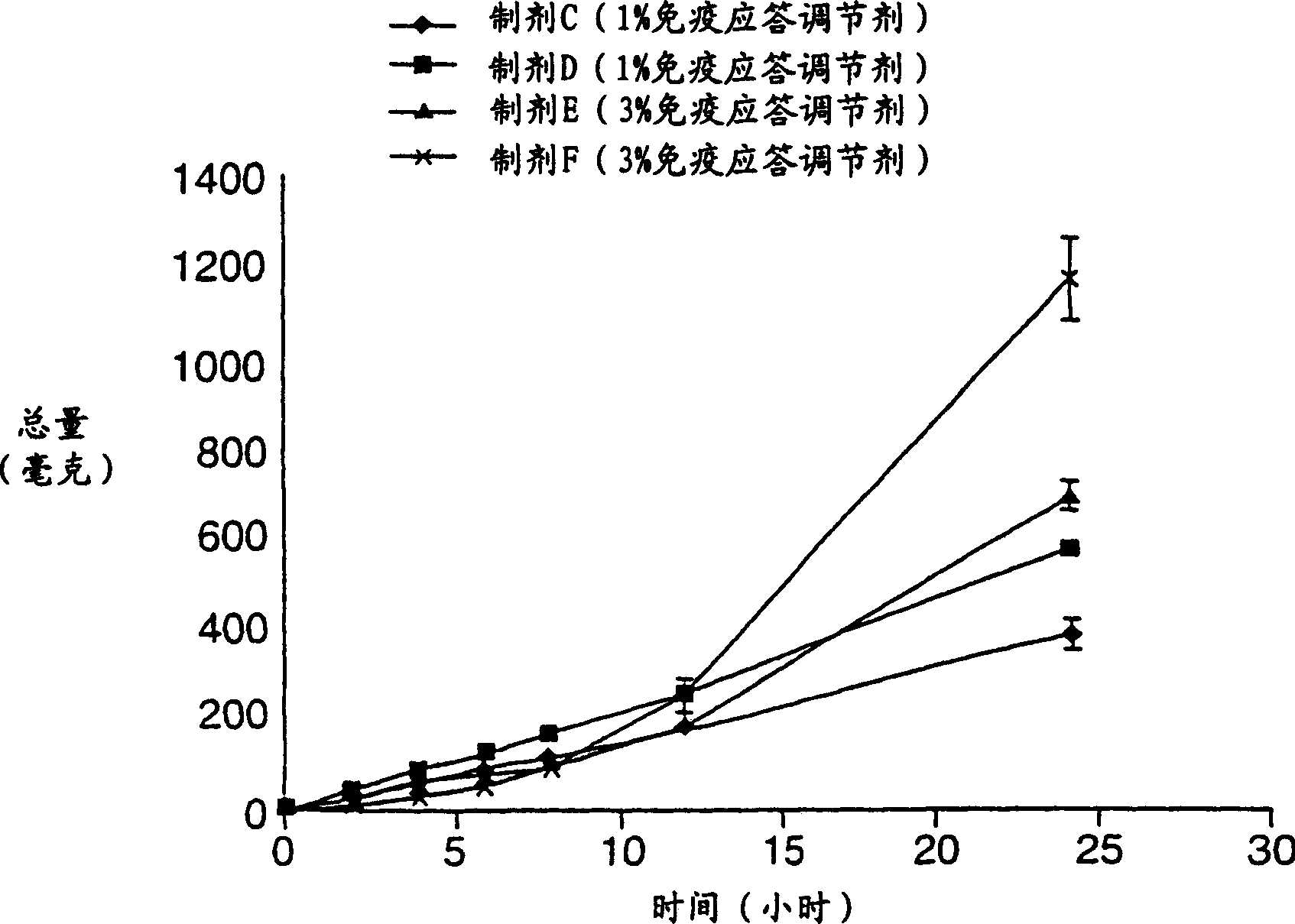
[0284] Example 3 Preparation of Pharmaceutical Formulations C-F
[0285] Pharmaceutical formulations C-F were prepared with the components in Table 3 below. Formulations C-F were prepared in the same manner as Formulation B in Example 2.
[0286] components
[0287] * PG is propylene glycol
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com