Preparing process of medicinal composition for treating acute and chronic bronchitis
A technology for chronic bronchitis and composition, applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., can solve the problems of uncontrollable preparation quality, inconvenient storage and taking, backward technology and dosage form, etc. Control quality, improve particle properties, and satisfy clinical absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 The preparation of pharmaceutical composition capsule of the present invention
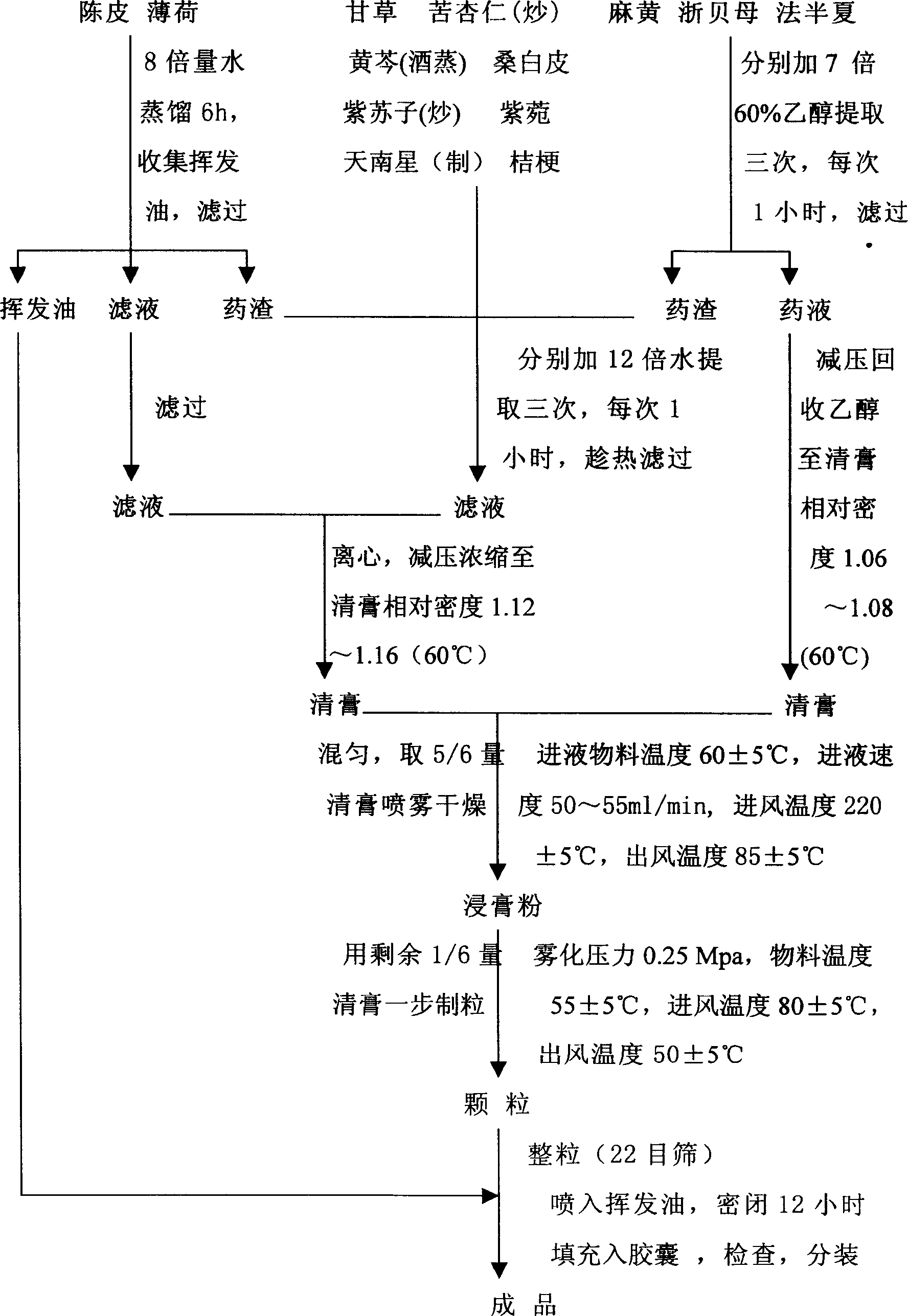
[0027] Ephedra 283g, licorice 226g, bitter almond (stir-fried) 142g, French pinellia 113g, scutellaria baicalensis (steamed with wine) 85g, Araceae (manufactured) 85g, perilla seed (stir-fried) 85g, mint 85g, bellflower 85g, Fritillaria 85g, 85g mulberry skin, 85g tangerine peel, 57g aster;
[0028] The above thirteen flavors, tangerine peel and peppermint add 8 times the amount of water to distill and extract the volatile oil for 6 hours, collect the volatile oil, filter, and the filtrate and medicinal residues are used for later use. Ephedra, Pinellia, and Fritillaria were respectively extracted with 8 times the amount of 60% ethanol (adjusted to PH=5 with 20% hydrochloric acid) for 3 times, each time for 1 hour, filtered (300 mesh), combined filtrates, and recovered under reduced pressure The relative density of ethanol Zhiqing ointment is 1.06~1.08 (60°C), for later use; t...
Embodiment 2
[0030] Embodiment 2 Preparation of pharmaceutical composition tablet of the present invention
[0031] Ephedra 466g, licorice 80-372g, fried bitter almonds 50-234g, French pinellia 40-186g, wine steamed scutellaria baicalensis 30-140g, made Aria 30-140g, fried perilla seeds 30-140g, mint 30-140g, bellflower 30g ~140g, Fritillaria 30~140g, Morus alba 30~140g, Tangerine peel 30~140g, Aster 20~94g;
[0032] The above thirteen flavors, tangerine peel and peppermint add 8 times the amount of water to distill and extract the volatile oil for 6 hours, collect the volatile oil, filter, and the filtrate and medicinal residues are used for later use. Ephedra, Pinellia, and Fritillaria were respectively extracted with 8 times the amount of 60% ethanol (adjusted to PH=5 with 20% hydrochloric acid) for 3 times, each time for 1 hour, filtered (300 mesh), combined filtrates, and recovered under reduced pressure The relative density of ethanol Zhiqing ointment is 1.06~1.08 (60°C), for later ...
Embodiment 3
[0033] Embodiment 3 The content control of the index component of the pharmaceutical composition of the present invention
[0034] Accurately weigh about 1.5 g of the drug content of the present invention prepared in Example 1, put it in a 25 ml measuring bottle, add an appropriate amount of methanol, ultrasonicate for 60 minutes, let it cool, add methanol to dilute to the mark, shake well, filter, discard the initial For the filtrate, accurately measure 10ml of the filtrate, put it on a water bath and evaporate to dryness, add methanol to the residue to dissolve it, and quantitatively transfer it to a 5ml volumetric flask, dilute to the mark, shake well, and use it as the test solution. Take another ephedrine hydrochloride reference substance, add methanol to make a solution containing 1.5mg per 1ml, as the reference substance solution. Test according to thin-layer chromatography (Appendix IVB, Chinese Pharmacopoeia, 2000 edition one), draw reference substance solution 1 μ l ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Tube chief | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com