Method for purifying (meth)acrylic ester
A technology of acrylate and refining method, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., to achieve the effect of great industrial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
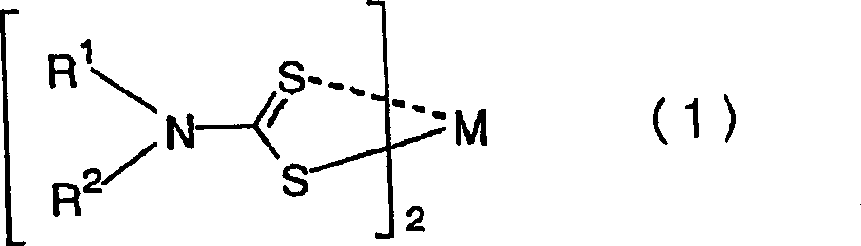
[0038] Weigh respectively the 2-ethylhexyl acrylate crude product 30g obtained above and the bis(di-n-butyldithiocarbamate) copper (II) equimolar with p-toluenesulfonic acid existing in the system, put Put it into a container with an inner volume of 100ml equipped with a thermocouple and a gas blowing tube. The container was immersed in an oil bath, and air was blown into the liquid at a rate of 15 ml / min. The liquid temperature in the container was kept at 130°C, and heating was continued for 8 hours. As a result of gas chromatographic analysis every hour, 2-ethylhexanol increased to 0.030% by weight within 1 hour from the start of heating, and no increase in the concentration of 2-ethylhexanol was observed for the next 8 hours.
Embodiment 2
[0042] Except for adding bis(di-n-butyldithiocarbamate) copper (II) in an amount 0.5 times the molar amount of p-toluenesulfonic acid, the result of the heating test was performed in the same manner as in Example 1. The 2-ethylhexanol concentration was 0.090% by weight. The concentration of this alcohol is within the allowable range as a product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com