Method for preparing organic pigment of red benzimidazolones
A technology of benzimidazolone and aminobenzimidazolone, which is applied in the field of preparation of red benzimidazolone organic pigments, and can solve problems such as difficult recycling and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0013] The preparation method of the red benzimidazolone organic pigment of the present invention, it comprises the steps:
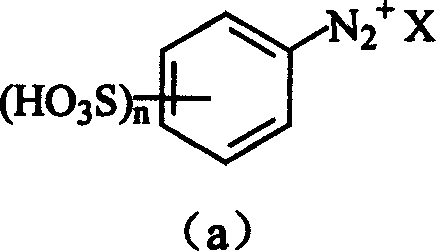
[0014] (1) the diazotization reaction of substituted aromatic primary amine and aminobenzenesulfonic acid:
[0015] This is an existing technology, which will not be repeated here, and its reaction formula is as follows:
[0016]
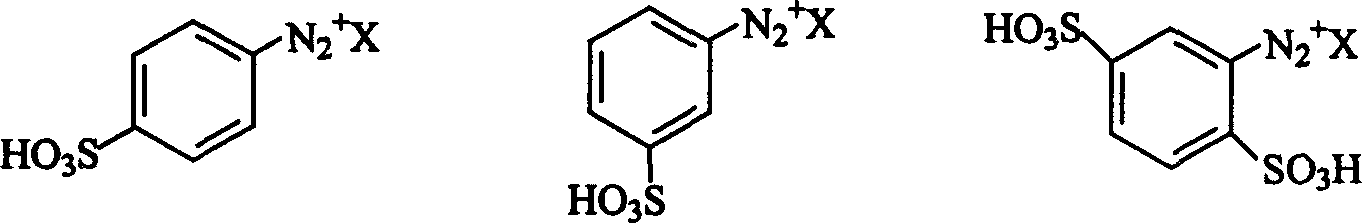
[0017] Where: X is Cl - or HSO 4 - ; Described substituted aromatic primary amine [compound (b)] is optional: o-fluoroaniline, o-chloroaniline, p-chloroaniline, m-bromoaniline, m-iodoaniline, o-toluidine, p-toluidine, o-methoxyaniline, o-Nitroaniline, m-nitroaniline, p-nitroaniline, methyl anthranilate, n-butyl anthranilate, 2,4-dimethylaniline, o-methyl-p-chloroaniline, 2-methyl -5-Chloroaniline, 2-methyl-4-nitroaniline, 2-methyl-4-amino-5-methoxy-benzenesulfonylmethylamine, 2-methoxy-4-chloroaniline, 2 -Methoxy-5-chloroaniline, 2-methoxy-4-nitroaniline, 2-nitro-4-methyl-aniline, 2-nitro-4-methoxyaniline, 2-nitro -4-...
Embodiment 1
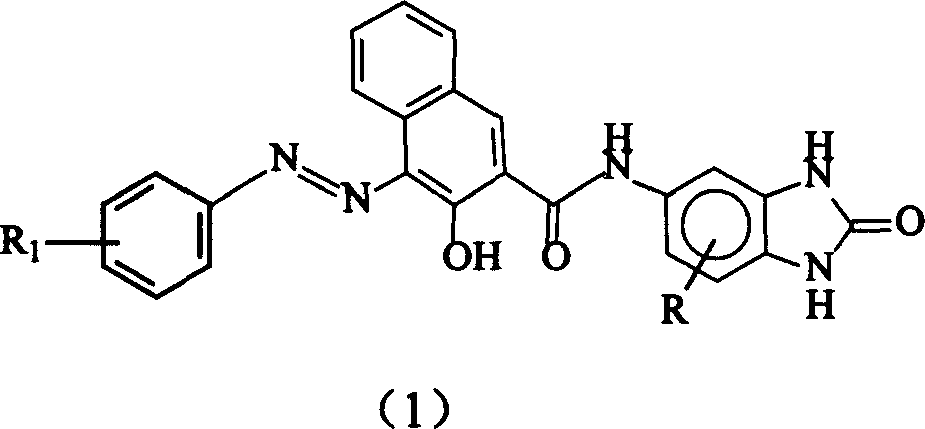
[0039] Preparation of n-butyl phthalate phenylazo-5-(2', 3'-hydroxynaphthoamide)-aminobenzimidazolone (C.I. Pigment Red 208)
[0040] The main reaction formula is as follows:
[0041]
[0042] Preparation of diazo liquid
[0043] 1) The diazotization of sulfamic acid
[0044] Para-aminobenzenesulfonic acid (1.73g, 10mmol) was dissolved in water (25ml, containing 10mmolNaOH), stirred at room temperature for 15min, filtered, discarded insolubles, cooled to 0-5°C in an ice bath, added NOHSO 4 (1.59 g, 12.5 mmol, composed of concentrated sulfuric acid and sodium nitrite), after the addition was completed, stirring was continued for 10 min to obtain a gray suspension, which was diluted with 1N hydrochloric acid to a total volume of 50 ml, and refrigerated for later use.
[0045] 2) The diazotization of n-butyl anthranilate
[0046] n-Butyl anthranilate (35g, 0.18mol), water (800ml) and 30% hydrochloric acid (30ml) were mixed and cooled to 0-5°C with an ice bath, a small amoun...
Embodiment 2
[0055] Preparation of n-butyl phthalate phenylazo-5-(2', 3'-hydroxynaphthoamide)-aminobenzimidazolone (C.I. Pigment Red 208)
[0056] The synthesis of the crude pigment is the same as that in Example 1.
[0057] Pigmentation processing of crude pigments
[0058] According to the weight of crude pigment (g): DMF (ml)=1:20, the dry crude pigment and solvent were added to the reactor, and after the addition was completed, the temperature was raised to 80-90° C. under stirring, and the mixture was kept stirring for 0.5-1 hour. Subsequently, the temperature was lowered to 15-20°C for filtration, the DMF mother liquor was recovered and used again, the filter cake was washed with water, dried and pulverized to obtain 65g of fine pigments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com