Preparation method and application of pH fluorescence probe
A fluorescent probe and fluorescent molecular probe technology, applied in the field of analytical chemistry, can solve the problems of cell apoptosis, cell dysfunction, organelle damage, etc., and achieve the effects of sensitive detection, novel principle and fast detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Preparation of PH fluorescent probe
[0070] A preparation method of PH fluorescent probe, the steps include:
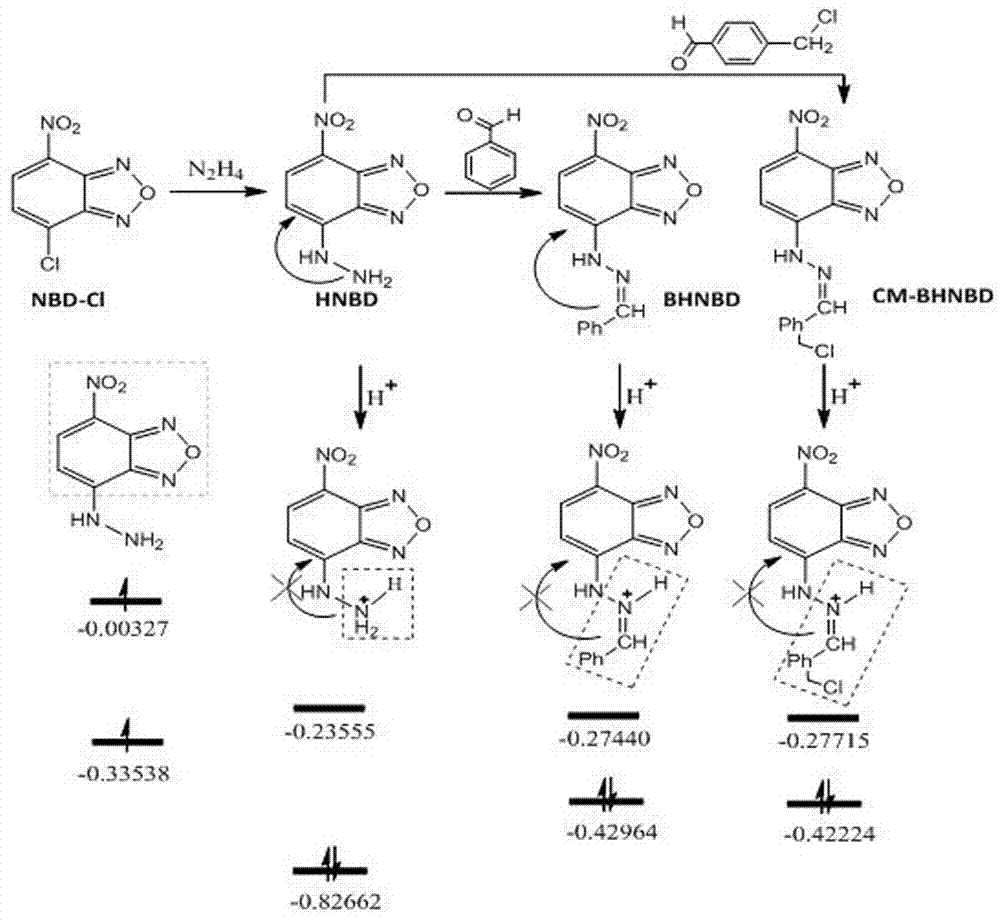
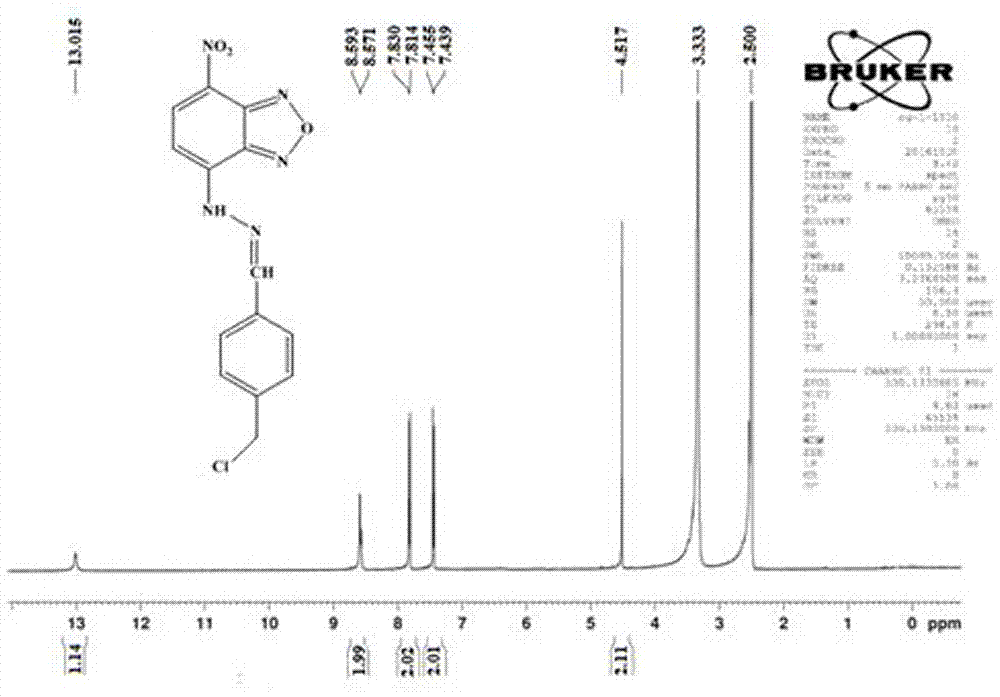
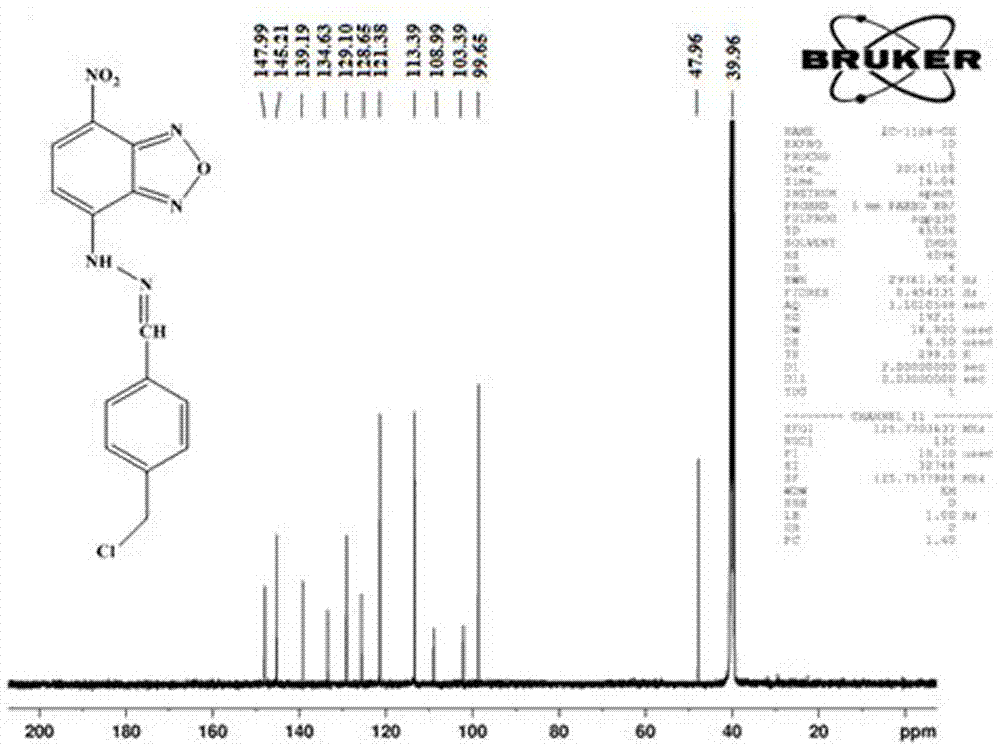
[0071] (1) Dissolve NBD-Cl (7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole) in chloroform at a concentration of 0.004g / ml, and then add the same volume of chloroform solution and chloroform solution Contain 1% hydrazine hydrate-methanol solution (in the hydrazine hydrate-methanol solution, the volume concentration of hydrazine hydrate is 1%), mix well, stir at room temperature for 2h to obtain brown precipitate, the precipitate is completely precipitated, filtered, the filter cake is passed through ethyl acetate Ester washing and drying to obtain NBD hydrazine;
[0072] (2) Add pyridinium chlorochromate to freshly distilled anhydrous dichloromethane, add 0.083 g / mL concentration, dissolve ultrasonically, and then add 4-(chloromethyl)benzyl alcohol with a concentration of 0.05 g / mL, The reaction was stirred at room temperature for 3 hours under nitrogen protect...
Embodiment 2
[0080] Example 2: Preparation of PH fluorescent probe
[0081] A preparation method of PH fluorescent probe, the steps include:
[0082] (1) Dissolve NBD-Cl (7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole) in chloroform at a concentration of 0.008g / mL, and then add the same volume of chloroform solution and chloroform solution Contain 1% hydrazine hydrate-methanol solution (in the hydrazine hydrate-methanol solution, the volume concentration of hydrazine hydrate is 1.2%), mix well, stir at room temperature for 2 h to obtain brown precipitate, filter, and wash the filter cake with ethyl acetate. Dry it to get NBD hydrazine;
[0083] (2) Add pyridinium chlorochromate to freshly distilled anhydrous dichloromethane, add 0.12g / ml concentration, dissolve by ultrasonic, then add 4-(chloromethyl)benzyl alcohol with a concentration of 0.08 g / mL, The reaction was stirred at room temperature under nitrogen protection for 4 h. After the reaction was completed, 3 times the volume of dichloromethane w...
Embodiment 3
[0085] Example 3: Preparation of PH fluorescent probe
[0086] A preparation method of PH fluorescent probe, the steps include:
[0087] (1) Dissolve NBD-Cl (7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole) in chloroform at a concentration of 0.001g / mL, and then add the same volume of chloroform solution to the chloroform solution Containing 0.39% hydrazine hydrate-methanol solution (in the hydrazine hydrate-methanol solution, the volume concentration of hydrazine hydrate is 0.2%), mix well, stir at room temperature for 2 h to obtain a brown precipitate, filter, and wash the filter cake with ethyl acetate. Dry it to get NBD hydrazine;
[0088] (2) Add pyridinium chlorochromate to dichloromethane, add concentration of 0.041g / ml, dissolve ultrasonically, then add 4-(chloromethyl)benzyl alcohol with a concentration of 0.02 g / mL, room temperature under nitrogen protection Stir the reaction for 3 hours. After the reaction is completed, add 4 times the volume of dichloromethane anhydrous diethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com