Antisense nucleic acid sequence for SARS coronavirus gene and its pharmaceutical use
A technology of antisense nucleic acid and coronavirus, which is applied in the field of antisense nucleic acid sequence against SARS coronavirus gene and its medicinal application, and can solve the problem that the specificity is not very high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Determination of the attack target of SA series antisense nucleic acid sequences and synthesis of antisense nucleic acid sequences.
[0043] Select the site of antisense nucleic acid attack on each gene segment of whole SARS coronavirus genetic map (about 30Kb).
[0044] The designed antisense nucleic acid was synthesized by the phosphoramidite solid-phase synthesis method using the Model 392 DNA-RNA synthesizer manufactured by APPLIEDBIOSYSTEMS and its supporting reagents, and then modified with thio. Synthetic raw materials are provided by synthesizer manufacturers, the main raw materials are: four diisopropyl β-cyanoethyl phosphoramidite monomers: adenosine (A), guanosine (G), thymidine (T), Cytosine (C); Four kinds of control pore glass powder (Control Pore Glass) solid-phase synthesis carrier (A, G, C, T); Activation reagent: tetrazole / acetonitrile capping reagent: acetic anhydride / dimethylpyrimidine / tetrahydrofuran, 1-methylpyrimidine / tetrahydrofuran;...
Embodiment 2
[0055] Embodiment 2: The chemical composition structure of SA series antisense nucleic acid sequence
[0056] Treat the synthesized and purified oligonucleotides with iodine to convert the phosphothioester bonds into phosphodiester bonds, and then use conventional DNA sequencing methods to sequence all antisense nucleic acid sequences, and the results show that the obtained antisense nucleic acids The sequences are consistent with the designed sequences; the molecular weights of all antisense nucleic acid sequences are determined by capillary electrophoresis, and found to be approximately equal to the theoretical molecular weights.
Embodiment 3
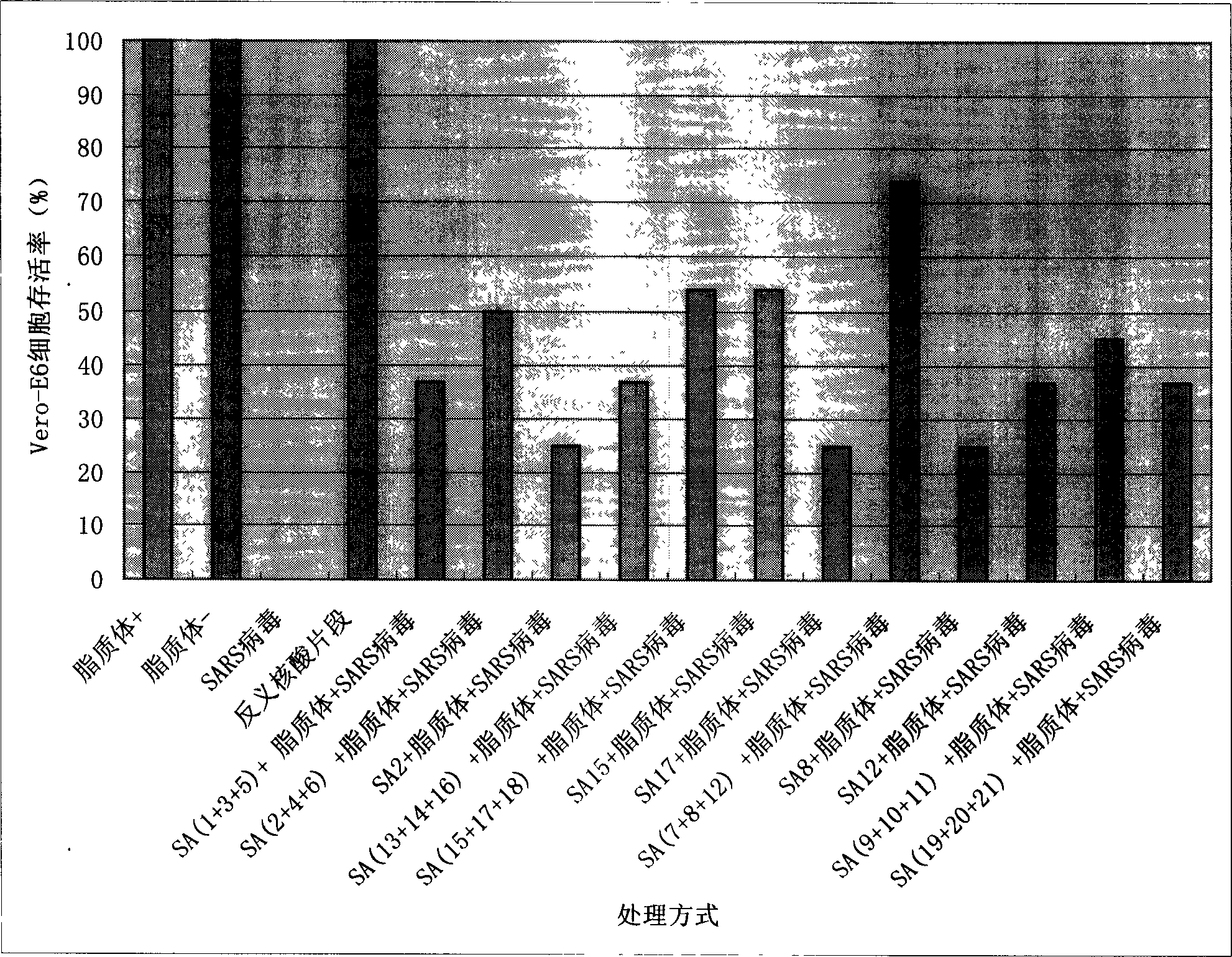
[0057] Embodiment 3: The inhibition of SA series antisense nucleic acid sequence for the African green monkey kidney cells infected with SARS:
[0058] 1. Verify with SARS virus-BJ-01 strain on the African green monkey kidney cell (Vero-E6) described in embodiment 3. Operated in P3 laboratory.
[0059] 2. The dose of SARS coronavirus is 10 -3 (Viral half infective dose TCID 50 =10 -7 , the virus dose used in this experiment is four orders of magnitude higher than the virus half infection dose), usually 2-3 days lesions.
[0060] 3. The dose of the drug is 1 μM
[0061] 4. Judging index (determination of efficacy by CPE method)
[0062] Cells without lesions:-
[0063] Cytopathy below 25%: +
[0064] Cytopathic in 26-50%: ++
[0065] Cytopathic in 51-75%: +++
[0066] Cytopathic at 76-100%: ++++
[0067] The calculation of cell viability was estimated according to the observation scale mentioned above. Cytopathy between + and +++ is calculated as the median of the cy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com