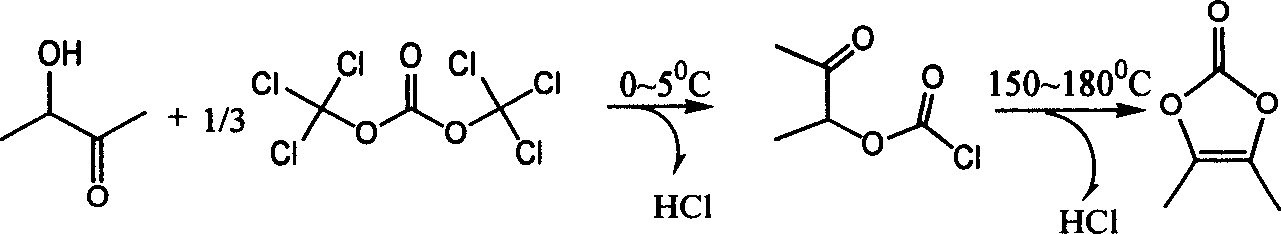
Synthesis of 4,5-dimethyl-1,3-dioxane amylene-2-one
A dioxole, chemical synthesis technology, applied in 4, can solve diphosgene refining difficulties, storage and transportation difficulties, safety hazards and other problems, to achieve great implementation value and social and economic benefits, low production cost, production Safe and reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] The molar ratio of feeding is: 3-hydroxy-2-butanone:bis(trichloromethyl)carbonate:auxiliary agent=1:0.33:1.0; the auxiliary agent is N-methylpyrrole. The organic solvent is carbon tetrachloride, and its consumption is 8 times of the quality of 3-hydroxyl-2-butanone;
[0010] Add 44g of 3-hydroxy-2-butanone, additives and organic solvents into a 500ml four-neck flask equipped with mechanical stirring, a constant pressure dropping funnel, a reflux condenser, and a thermometer. Under stirring, cool to 0°C, and then drop Add an organic solvent solution of bis(trichloromethyl)carbonate, and stir the reaction at 0-5°C for 2-3 hours. After the organic solvent is recovered by distillation under reduced pressure, an oily liquid is obtained, and then the temperature is slowly raised to 160°C. Stir at -165°C for 60 minutes, cool to below 25°C, add 160ml of water and 300ml of ether, stir for 15 minutes to separate the layers, extract the water layer with an appropriate amount of et...
Embodiment 2
[0012] The molar ratio of feeding is: 3-hydroxy-2-butanone:bis(trichloromethyl)carbonate:auxiliary agent=1:0.33:1.0; the auxiliary agent is N-methylpyrrole. The organic solvent is carbon tetrachloride, and its consumption is 8 times of the quality of 3-hydroxyl-2-butanone;
[0013] Under the protection of nitrogen, 3-hydroxy-2-butanone (44g), additives and organic solvents were successively added to a 500ml four-neck flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, and opened Stir and cool to 0°C under stirring, then add bis(trichloromethyl)carbonate organic solvent solution dropwise at 0-5°C. After the addition, continue to stir and react at 0-5°C for 2 hours, distill under reduced pressure to recover the organic solvent, then slowly heat the obtained oily liquid, and stir at 165-170°C for 60 minutes, cool to below 25°C, add 160ml of water and 300ml of diethyl ether, stirred for 15 minutes to separate the layers, t...
Embodiment 3
[0015] The molar ratio of feeding is: 3-hydroxy-2-butanone:bis(trichloromethyl)carbonate:auxiliary agent=1:0.35:1.1; the auxiliary agent is N-methylpyrrole. The organic solvent is carbon tetrachloride, and its consumption is 10 times of the quality of 3-hydroxyl-2-butanone;
[0016] Under the protection of nitrogen, 3-hydroxy-2-butanone (44g), additives and organic solvents were successively added to a 500ml four-neck flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, and opened Stir and cool to 0°C under stirring, then add bis(trichloromethyl)carbonate organic solvent solution dropwise at 0-5°C. After the addition, continue to stir and react at 0-2°C for 2 hours, recover the organic solvent by distillation under reduced pressure, then slowly heat the obtained oily liquid, and stir at 150-160°C for 75 minutes, cool to below 25°C, add 160ml of water and 300ml of diethyl ether, stirred for 15 minutes to separate the lay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com