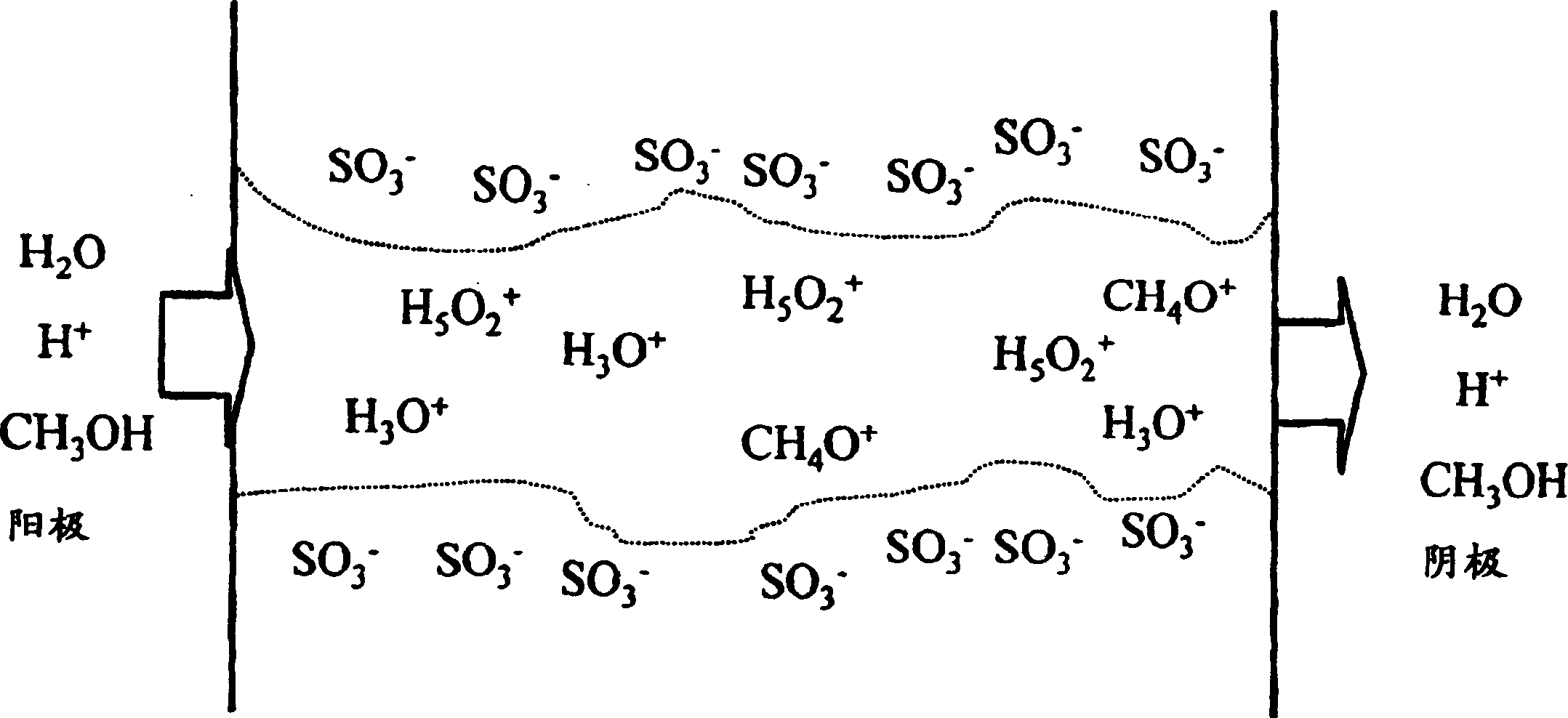
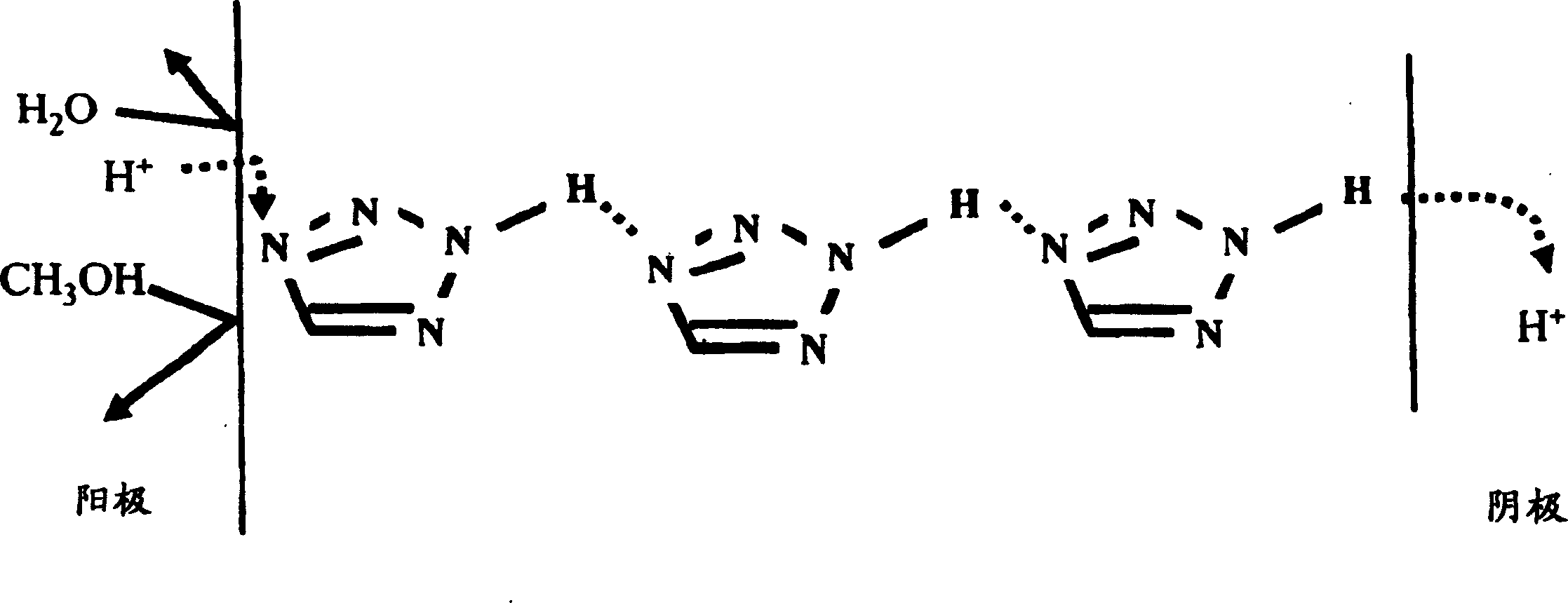
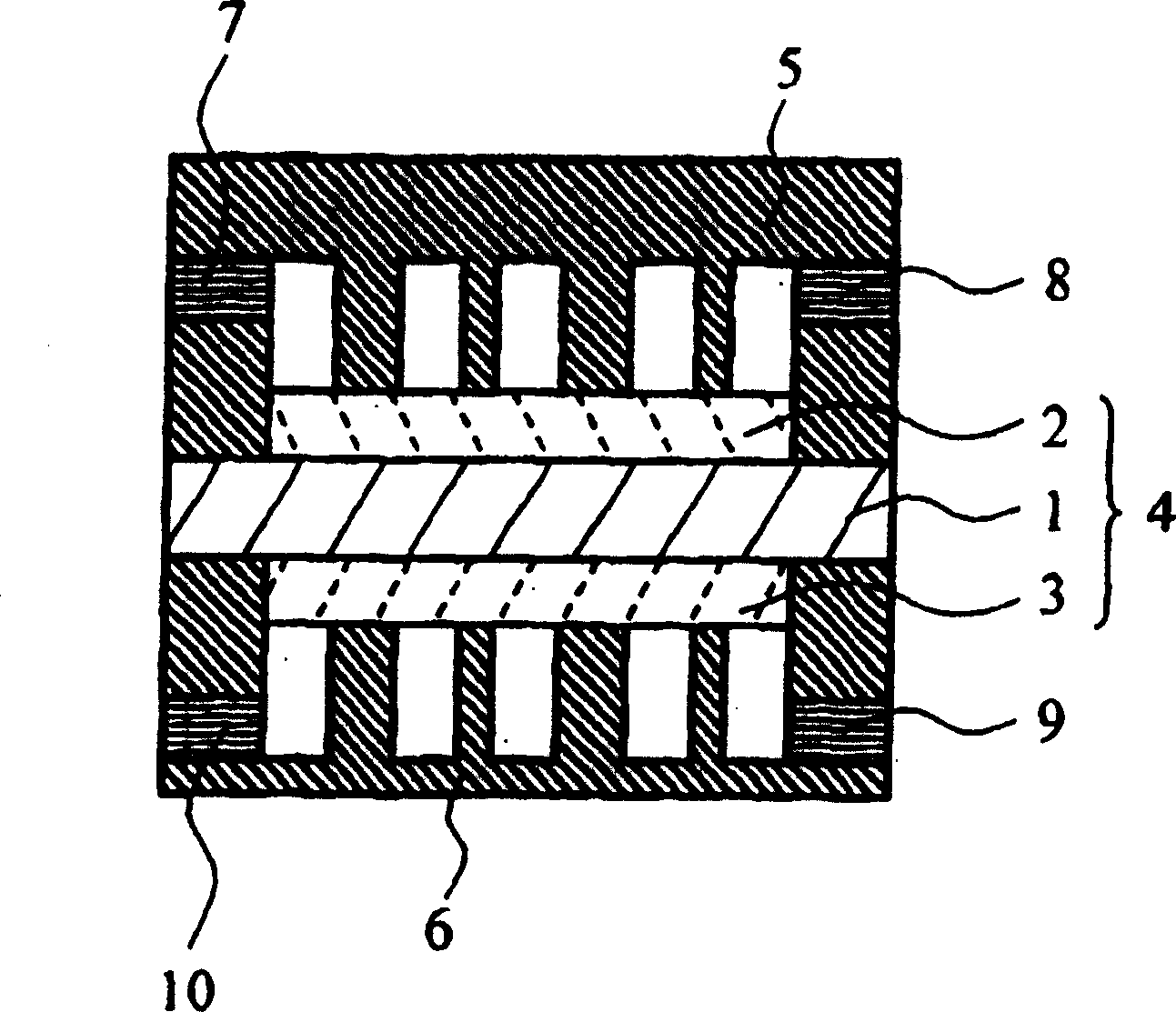
Proton conductivity polymer, catalyst compsn.electrolytic memberane for fuel cell and fuel cell
一种质子传导性、燃料电池的技术,应用在固体电解质燃料电池、燃料电池、燃料电池助剂等方向,能够解决质子导电性降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] 2.00 g of 3-acrylamido tetrazole and 0.0293 g of azobisisobutyronitrile were dissolved in 20 ml of DMF (N,N-dimethylformamide). Then, Ar was blown into the mixture to perform gas replacement, and the mixture was reacted at 65° C. for 15 hours under an Ar atmosphere. After concentrating the reaction mixture, it was dropped into methanol, and the precipitate was filtered and dried to obtain a polymer. MW=16000. The structural formula of this polymer is shown below.
[0136]
Embodiment 2
[0138] 2.00 g of 3-acrylamido tetrazole, 1.50 g of styrene, and 0.0289 g of azobisisobutyronitrile were dissolved in 20 ml of DMF, and Ar was blown into the mixture for replacement. Under an Ar atmosphere, at 65 It was reacted at ℃ for 15 hours. After concentrating the reaction mixture, it was dropped into methanol, and the precipitate was filtered and dried to obtain a polymer. MW=26000. The structural formula of this polymer is shown below. At this time, the feeding ratio is m:n=1:1.
[0139]
Embodiment 3
[0141] 2.00 g of 3-acrylamido tetrazole, 0.763 g of acrylonitrile and 0.0310 g of azobisisobutyronitrile were dissolved in 20 ml of DMF, and Ar was blown into the mixture to replace it. Under Ar atmosphere, at 65 It was reacted at ℃ for 15 hours. After concentrating the reaction mixture, it was dropped into methanol, and the precipitate was filtered and dried to obtain a polymer. MW=31000. The structural formula of this polymer is shown below. At this time, the feeding ratio is m:n=1:1.
[0142]
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com