Rapid detection of bt-cry toxins
A toxin and rapid immunization technology, which is applied in the direction of measuring devices, biological tests, material inspection products, etc., can solve the problem of expensive immune chromatographic strips
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
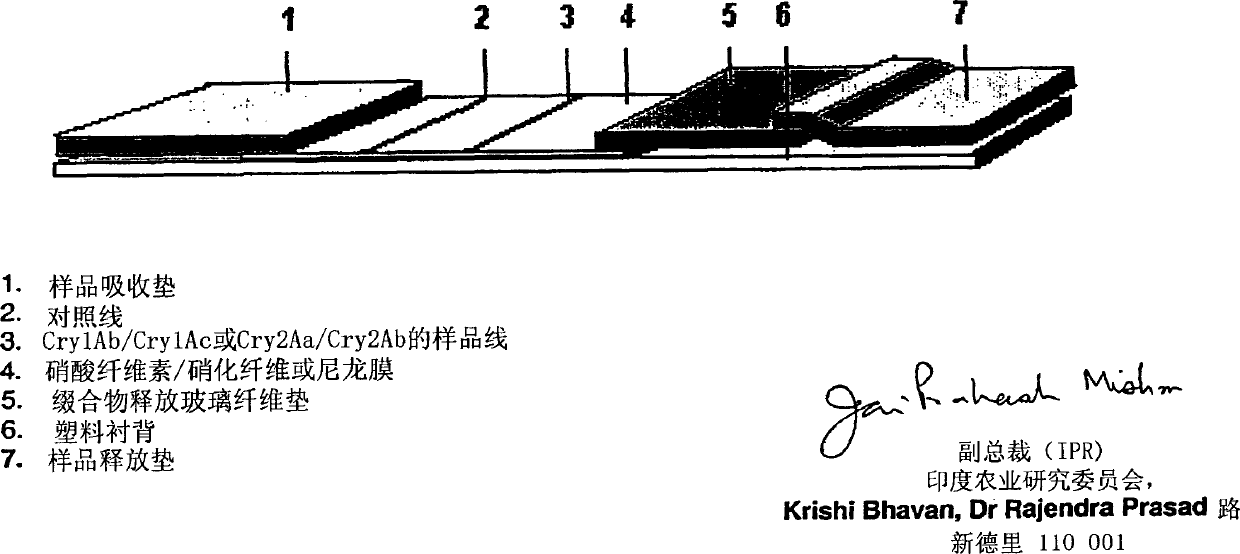
Image
Examples
Embodiment (1
[0013] Example (1): Preparation of Rapid Immunochromatographic Assay / Band to Detect Cry1Ac
[0014] 1. Isolation of Cry1Ac from clones, which is done by ultrasonically degrading bacterial clone cultures, and sedimentation to remove cell debris and insoluble toxins. The toxin is dissolved in alkaline buffer and extracted by centrifugation. Toxin purification was accomplished by 25% saturated ammonium sulfate precipitation and polyacrylamide electrophoresis.
[0015] 2. Antiserum against Cry1Ac toxin was raised in rabbits or goats by injecting purified toxins respectively.
[0016] 3. The method of culturing antiserum is to initially inject the antigen (Cry1Ac) mixed with Freund's complete adjuvant and repeated booster doses mixed with Freund's incomplete adjuvant, and then collect the serum. This common technique can be obtained from the immunology textbook ( Antibodies-A laboratory Manual - Harlow Ed and David Lane; Cold Spring Harbor Laboratory, USA, 1988) and will not be d...
Embodiment (2
[0036] Example (2): Preparation of a rapid immunochromatographic assay / band to detect Cry1Ac / Cry2Ab using Cry-toxin receptor protein as capture ligand.
[0037] 1. Isolation of Cry1Ac and Cry2Aa / Cry2Ab from clones, which is by ultrasonically degrading bacterial clone cultures, and sedimentation to remove cell debris and insoluble toxins. The toxin is dissolved in alkaline buffer and extracted by centrifugation. Toxin purification was accomplished by 25% saturated ammonium sulfate precipitation and polyacrylamide electrophoresis.
[0038] 2. Cultivate antisera against Cry1Ac / Cry2Aa / Cry2Ab toxins in rabbits or goats by injecting purified toxins, respectively.
[0039] 3. The method of raising antiserum is initial injection of antigen (Cry1Ac / Cry2Aa / Cry2Ab) mixed with Freund's complete adjuvant and repeated booster doses mixed with Freund's incomplete adjuvant, and then the serum is collected. This general technique can be obtained from Immunology textbook ("Antibodies - A Labo...
Embodiment (3
[0060] Example (3): Preparation of a rapid immunochromatographic assay / strip to simultaneously detect Cry1Ac and Cry2Ab.
[0061] 1. Isolation of Cry1Ac and Cry2Aa / Cry2Ab from clones, which is by ultrasonically degrading bacterial clone cultures, and sedimentation to remove cell debris and insoluble toxins. The toxin is dissolved in alkaline buffer and extracted by centrifugation. Toxin purification was accomplished by 25% saturated ammonium sulfate precipitation and polyacrylamide electrophoresis.
[0062] 2. Cultivate antisera against Cry1Ac / Cry2Aa / Cry2Ab toxins in rabbits or goats by injecting purified toxins, respectively.
[0063] 3. The method of raising antiserum is initial injection of antigen (Cry1Ac / Cry2Aa / Cry2Ab) mixed with Freund's complete adjuvant and repeated booster doses mixed with Freund's incomplete adjuvant, and then the serum is collected. This general technique can be obtained from Immunology textbook ("Antibodies - A Laboratory Manual" - Harlow Ed and Da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com