Hydrophobically modified succinylated chitosan derivative and its prepn process
A technology of succinylated chitosan and hydrophobic modification, which is applied in the field of biological and medical polymer materials, can solve the problems of poor water solubility, no solubilization and emulsifying ability of chitosan, and achieves good product quality and simple preparation method. Ease of operation, the effect of reducing surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
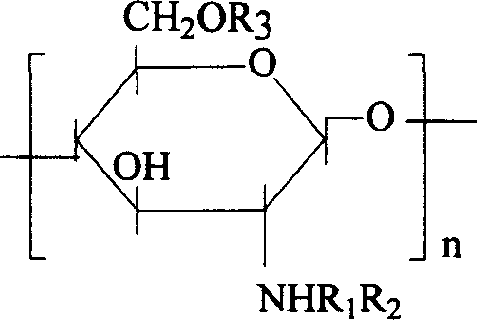
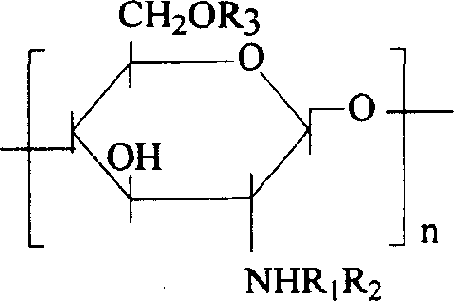
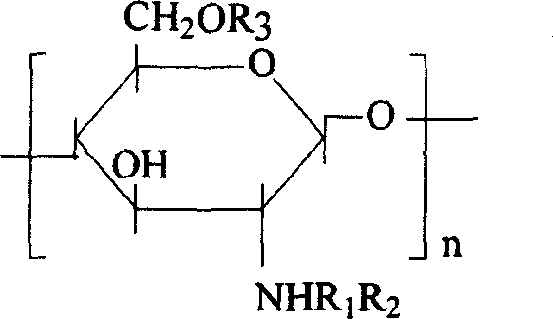
[0019] Embodiment 1: the succinylated chitosan derivative of hydrophobic modification can be expressed as:
[0020]
[0021] where R 1 for H / COCH 3 , the degree of deacetylation is 90%, R 2 , R 3 COCH 2 CH 2 COO - or CH 2 CHOHCH 2 O(CH 2 ) 3 CH 3 .
[0022] The preparation method is as follows:
[0023] Dissolve 0.5g of chitosan with 25ml of 2% acetic acid, then add 25ml of methanol, stir evenly and add 0.5g of succinic anhydride to react at 30°C for 5h. The product is thoroughly soaked in ethanol for dehydration, then washed with aqueous ethanol and acetone, and then vacuum-dried. Disperse the above-prepared succinylated chitosan in 0.15 g of KOH in 15 ml of isopropanol and alkalinize it for 4 hours, then add 3.3 g of butyl glycidyl ether dropwise into the flask using a partial pressure dropping funnel. Reflux and condense in a water bath at 55°C for 20 hours, then cool to room temperature. Cool to room temperature, neutralize with acetic acid, filter, wash ...
Embodiment 2
[0025] The general formula of the hydrophobically modified succinylated chitosan derivatives of the present embodiment is as in Example 1, the difference is that the degree of deacetylation is 80%, R 2 , R 3 COCH 2 CH 2 COO - or CH 2 CHOHCH 2 O(CH 2 ) 11 CH 3 .
[0026] The preparation method is as follows:
[0027] Dissolve 6g of chitosan with 250ml of 2% acetic acid solution, then add 250ml of methanol, stir evenly and add 100g of succinic anhydride to react at 35°C for 3h. The product is thoroughly soaked in ethanol for dehydration, then washed with aqueous ethanol and acetone, and then vacuum-dried. The succinylated chitosan prepared above was dispersed in 200ml of isopropanol solution of 1% KOH and then alkalized for 5 hours, and 15g of dodecyl glycidyl ether was added dropwise into the flask with a partial pressure dropping funnel. After reacting in a 60°C water bath for 24 hours, it was cooled to room temperature. Neutralize with acetic acid, filter, wash wi...
Embodiment 3
[0029] The hydrophobically modified succinylated chitosan derivative of the present embodiment is as in Example 1, the difference is that the degree of deacetylation is 75%, R 2 , R 3 COCH 2 CH 2 COO - or (CH 2 ) 7 CH 3 .
[0030] The preparation method is as follows: dissolve 0.5g of chitosan with 25ml of 2% acetic acid, then add 25ml of methanol, stir evenly, add 0.5g of succinic anhydride and react at 25°C for 5h. The product is thoroughly soaked in ethanol for dehydration, then washed with aqueous ethanol and acetone, and then vacuum-dried. Disperse the above-prepared succinylated chitosan in 0.1 g of KOH in 15 ml of isopropanol solution and alkalize it for more than 4 hours. Take 2.9 g of bromooctane and add it dropwise into the flask with a partial pressure dropping funnel. After reacting in a 60°C water bath for 5 hours, it was cooled to room temperature. Neutralize with acetic acid, filter, wash with aqueous ethanol and acetone successively, and dry. The prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com