Levo carnitine dropping pill and preparation thereof
A technology of L-carnitine and L-carnitine tartrate, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, can solve problems such as the lack of public technical materials for L-carnitine dropping pills, and avoid liver and intestine problems. First-pass effect, improved bioavailability, simple equipment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] Now with several groups of specific examples, the preparation method of L-carnitine dripping pills of the present invention will be further described.
[0042] [The first group: L-carnitine dripping pills prepared with L-carnitine as the active ingredient]
[0043] 1. The embodiment using a single matrix
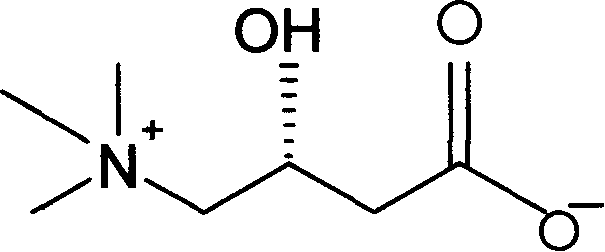
[0044] 1.1 English name of L-carnitine - L-carnitine, Chinese translation - Kang Liting;
[0045] Alias - L-carnitine, vitamin B T ;
[0046] Chemical name - the chemical name is L-β-hydroxy-γ-methylammonium butyric acid;
[0047] 1.2 Substrate - polyethylene glycol (2000~20000) , polyoxyl 40 stearate, beta cyclodextrin, poloxamer, sodium carboxymethyl starch, sodium lauryl sulfate, stearic acid, sodium stearate, glycerin gelatin, shellac;
[0048] 1.3 Ratio - in g or kg, L-carnitine: matrix = 1:1 ~ 1:5;
[0049] 1.4 According to the steps of [Preparation Method] 4-7, L-carnitine dripping pills of different specifications can be prepared.
[0050] [test resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com