Methods for producing mammalian trypsins
A trypsin and mammalian technology, applied in the field of preparation of mammalian trypsin, can solve the problems that the expression of mammalian trypsin has not been realized at the commercial level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1: Construction of pCaHj522
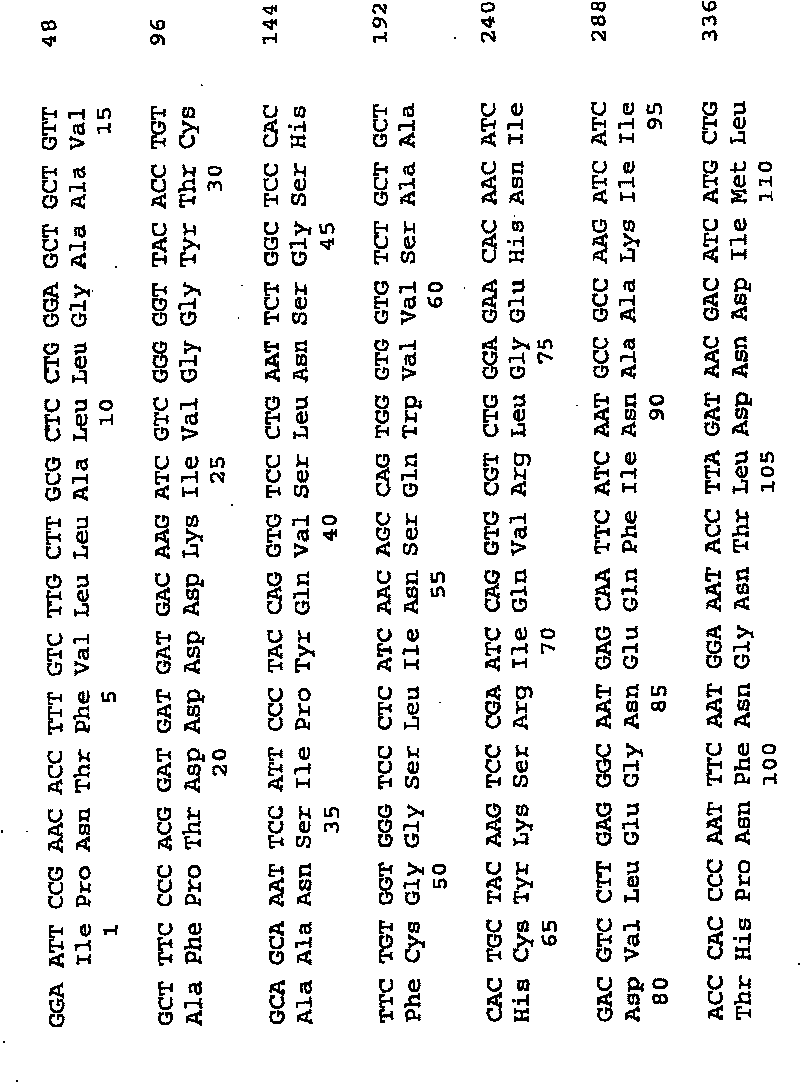
[0105] The porcine trypsinogen DNA sequence and deduced amino acid sequence are shown in Figure 2 (SEQ ID NO: 3 and 4, respectively). The porcine trypsinogen gene was cloned as a cDNA gene on plasmid p185 as described in WO 97 / 00316. The cDNA gene is fused with a TAKA amylase gene fragment encoding a TAKA amylase signal sequence in frame, and cloned into an expression plasmid of Aspergillus oryzae. The TAKA amylase gene fragment encoding the TAKA amylase signal sequence was amplified by PCR from plasmid pTAKA17, see EP 0 238 023. The Aspergillus expression vector pCaHj483 used is described in WO 98 / 00529. Fusion of the gene segment encoding the TAKA amylase signal sequence to the segment encoding porcine protrypsin was performed using splicing by overlap extension (Horton et al., 1989, Gene 77:61-68). Pwo DNA polymerase (Roche Molecular Biochemicals, Basel, Switzerland) was used for PCR amplification according to the manufacturer...
Embodiment 2
[0112] Example 2: Construction of expression vector pRaMB58
[0113] Construction of plasmid pRaMB58 ( Figure 4 ) to express porcine trypsin in Fusarium venenatum. First, the DNA fragment encoding the signal peptide and the propeptide of the Fusarium oxysporum trypsinogen-like protein (Figure 1; nucleotides 58-129 of SEQ ID NO: 1) was passed as a template using pJRoy6 (US Patent 5,837,847) by the following PCR primers for amplification:
[0114] Primer 980173: 5'-CTTCACCATGGTCAAGTTCGCT-3' (SEQ ID NO: 9)
[0115] Primer 980174: 5'-GTTGGGGATCTCCTGAGGAGCG-3' (SEQ ID NO: 10)
[0116] Second, the eDNA fragment (nucleotides 75-744 of SEQ ID NO: 3) encoding mature porcine trypsin shown in Figure 2 was amplified using pCaHj522 and the following PCR primers:
[0117] Primer 980175: 5'-ATCGTCGGGGGTTACACCTGT-3' (SEQ ID NO: 11)
[0118] Primer 980176: 5'-CGTTAATTAATTCTTTAGTTGGCAGCGATGGTCTG-3' (SEQ ID NO: 12)
[0119] All PCR reactions were performed using Pwo DNA polymerase with hi...
Embodiment 3
[0121] Example 3: Transformation of Fusarium venenatum with pRaMB58
[0122] Fusarium venenatum MLY-3 ([Delta]tri5) was obtained as described in US Patent 6,180,366. Spores of Fusarium venenatum MLY-3 were produced as follows: by inoculating flasks containing 500 ml RA sporulation medium with 10 plugs from 1X Vogels plates (2.5% Noble agar) supplemented with 2.5% glucose and 2.5 mM sodium nitrate, Incubate at 28°C, 150 rpm for 2-3 days. by MIRACLOTH TM (Calbiochem, San Diego, CA) and centrifuge at 7000 rpm for 20 minutes in a Sorvall RC-5B centrifuge (E.I. DuPont De Nemours and Co., Wilmington, DE). Precipitated spores were washed twice with sterile distilled water, resuspended in a small amount of water, and counted using a hemocytometer.
[0123] Protoplasts were prepared as follows: use 4 × 10 7 Fusarium venenatum MLY-3 spores were inoculated into 100 ml of YEPG and incubated at 24°C, 150 rpm for 16 hours. Centrifuge at 3500 for 7 minutes in a Sorvall RT 6000D (E.I. D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com