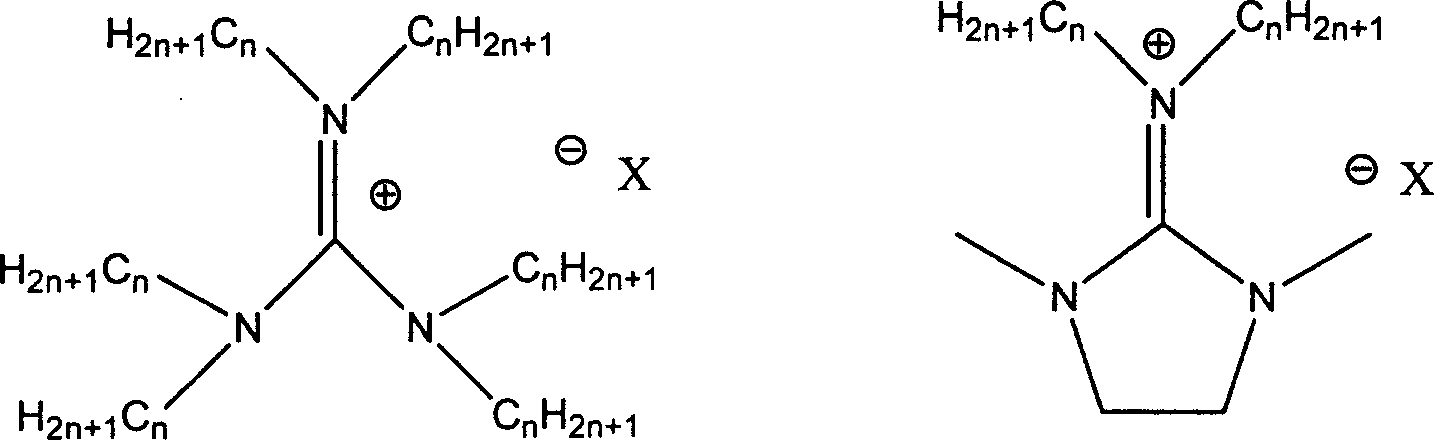Method for synthesizing cyclic carbonate
A technology for cyclic carbonates and compounds, which is applied in the field of synthesizing cyclic carbonates, can solve the problems of insufficient catalytic activity, unstable catalyst air, and difficult separation of catalysts, and achieve wide application range, easy recovery and recycling, high The effect of catalytic activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
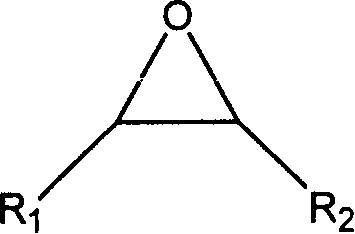
[0023] In a 100 ml autoclave, add zinc bromide 0.00002mol, 0.00012mol of guanidinium salt successively, the guanidine salt is: hexabutylguanidine bromide (a), hexapropylguanidine bromide (b), tetraethyl dibutyl guanidine bromide (c), tetramethyldibutylguanidine bromide (d), hexaisopropylguanidine bromide (e), tetraethyldihexylguanidine bromide (f), hexahexylguanidine bromide ( g), hexapentylguanidine bromide (h), hexaethylguanidine bromide (i), hexabutylguanidine chloride (j), hexabutylguanidine iodide (k), N, N'-dimethyl N, N'-ethylene, one of N"-dibutylguanidine salts (l), propylene oxide 0.172mol, the temperature is controlled by a temperature controller to slowly rise to a reaction temperature of 130°C, and a carbon dioxide pressure of 3MPa After one hour of reaction time, it was cooled to room temperature, and excessive carbon dioxide was slowly emitted, and the liquid obtained by the reaction was distilled under reduced pressure to obtain propylene carbonate, and the sel...
Embodiment 2
[0026] In a 100 milliliter autoclave, add zinc bromide 0.00002mol successively, 0.00012mol hexabutylguanidine bromide, propylene oxide 0.215mol, control at different reaction temperatures and carbon dioxide pressure (see Table 2), after reaction time one hour , cooled to room temperature, slowly emitting excess carbon dioxide, the liquid obtained by the reaction was distilled out of propylene oxide by normal pressure, and then distilled under reduced pressure to obtain propylene carbonate, the selectivity was 99%, the results are shown in Table 2.
[0027] Numbering
Embodiment 3
[0029] In a 100 ml autoclave, 0.00002 mol of zinc bromide, different proportions of hexabutylguanidine bromide, and 0.172 mol of propylene oxide were successively added, and the temperature was controlled by a temperature controller to slowly rise to a reaction temperature of 100°C and a carbon dioxide pressure of 4 MPa. After the set time (see Table 3), cool to room temperature, slowly emit excess carbon dioxide, and distill the liquid obtained from the reaction to distill propylene oxide under normal pressure, then distill under reduced pressure to obtain propylene carbonate, the selectivity is 99% %, and the results are shown in Table 3.
[0030] Numbering
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com