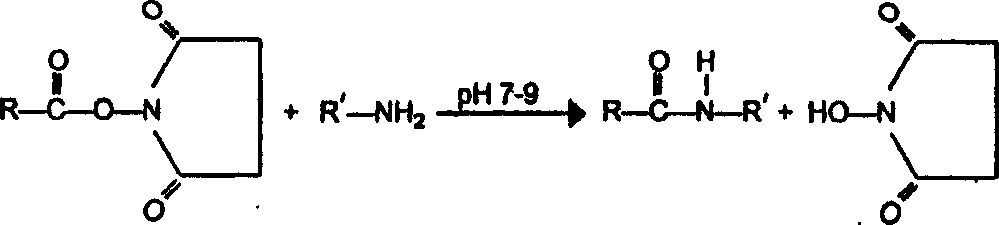Long lasting fusion peptide inhibitors of viral infection
An anti-virus, measles virus technology, applied in the direction of viral peptides, anti-viral agents, viruses, etc., can solve problems such as reducing effective anti-viral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0179] A particularly preferred method of preparing the compounds of the invention involves solid phase peptide synthesis in which the alpha-N-terminus of the amino acid is protected with an acid- or base-sensitive group. Such protecting groups should be stable to the conditions of peptide bond formation while being readily removable without disrupting the extended peptide chain or racemizing any chiral centers contained therein. Suitable protecting groups are: 9-fluorenylmethoxycarbonyl (Fmoc), tert-butoxycarbonyl (Boc), benzyloxycarbonyl (Cbz), biphenylisopropoxycarbonyl, tert-amyloxycarbonyl, Isobornyloxycarbonyl, α,α-dimethyl-3,5-dimethoxybenzyloxycarbonyl, o-nitrophenylsulfinyl, 2-cyano-tert-butoxycarbonyl and the like. The 9-fluorenyl-methoxycarbonyl (Fmoc) protecting group is particularly suitable for the synthesis of the peptides of the invention. Other preferred side chain protecting groups are: for side chain amino groups such as lysine and arginine, 2,2,5,7,8-penta...
Embodiment 1
[0221] Preparation of Modified DP178--
[0222] YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWFK(MPA)-NH 2 Synthesis
[0223] In this example, DP178 (SEQ ID NO: 1) was synthesized and modified to contain a linker and a maleimide group according to the following synthetic route. As described in U.S. Patents 6,013,236 and 6,020,459, DP178 is a potent inhibitor of HIV-1 and is capable of inhibiting cell-induced syncytium formation between HIV-1-infected and uninfected cells and the destruction of uninfected cells by cell-free HIV-1 virus infection.
[0224] Solid phase peptide synthesis of modified peptides on a 100 μmole scale was performed using manual solid phase synthesis, Symphony Peptide Synthesizer and Fmoc protected Rink amide MBHA resin. Add the following protected amino acids to the resin in sequence: Fmoc-Lys(Aloc)-OH, Fmoc-Phe-OH, Fmoc-Trp(Boc)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Trp(Boc)-OH , Fmoc-Leu-OH, Fmoc-Ser(tBu)-OH, Fmoc-Ala-OH, Fmoc-Trp(Boc)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Asp(tB...
Embodiment 2
[0228] Preparation of Modified DP107--
[0229] NNLLRAIEAQQHLLQLTVWQIKQLQARILAVERYLKDQK(MPA)NH 2 Synthesis
[0230] In this example, DP107 (SEQ ID NO: 2) was synthesized and modified to contain a linker and a maleimide group according to the following synthetic route. DP107 exhibits potent antiviral activity against HIV-1 as described in US Patent Nos. 6,013,236 and 6,020,459.
[0231] Solid-phase peptide synthesis of modified peptides on a 100 μmole scale was performed using manual solid-phase synthesis, a co-peptide synthesizer, and Fmoc-protected Rink amide MBHA resin. Add the following protected amino acids to the resin in sequence: Fmoc-Lys(Aloc)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Asp(tBu)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Leu-OH , Fmoc-Tyr(tBu)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Val-OH, Fmoc-Ala-OH, Fmoc-Leu-OH, Fmoc-Ile- OH, Fmoc-Arg(Pbf)-OH, Fmoc-Ala-OH, Fmoc-Gln(Trt)-OH, Fmoc-Leu-OH, Fmoc-Gln(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Ile-OH, Fmoc-Gln(Trt)-OH, Fmoc-Trp(Boc)-OH, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com