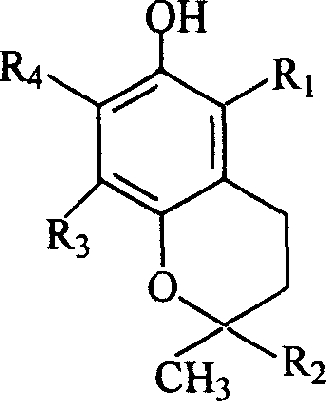
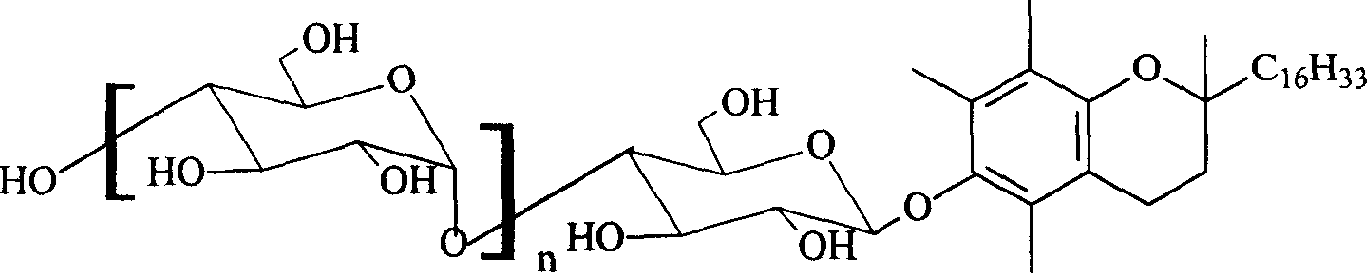
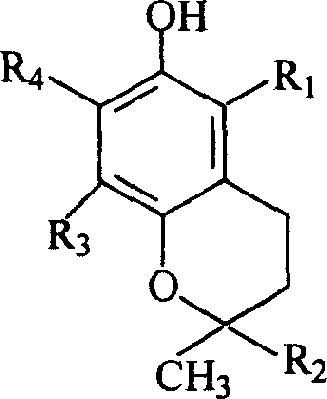
Benzo-dihydropyran glycoside derivatives
A technology of chroman and derivatives, which is applied in the field of chroman derivatives, and can solve the problems of vitamin E reduction, low activity, and loss of antioxidant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Synthesis of Compound 1 and Compound 3:
[0070] (1) Synthesis of 1,4-p-methoxy-2,3-dimethylbenzene:
[0071] 2,3-Dimethylhydroquinone (1.38g, 0.01mol) was dissolved in 8mL dimethylsulfoxide (DMSO), and methyl iodide (3.2mL, 7.3g, 0.05mol) and KOH (4g, 0.07mol) solid, reacted at room temperature for 5 hours, and thin-plate chromatography (TLC) showed that the reaction had ended. Add 20 mL of water and stir for 5 minutes, then add CH 2 Cl 2 Extracted several times, combined the dichloromethane extracts, successively washed with saturated Na 2 S 2 o 3 Wash with saturated solution and saturated brine, and dry over anhydrous magnesium sulfate. Filtration, the organic phase was evaporated under reduced pressure to remove the solvent, the concentrate was separated through a short column, and the 2 Cl 2 A mixed solvent (volume ratio of 2:1) was eluted, and the eluent was evaporated under reduced pressure to remove the solvent to obtain white crystal 1,4-p-methoxy-2,3-d...
Embodiment 2
[0128] Synthesis of Compound 2 and Compound 4:
[0129] (1) Synthesis of 1,4-p-methoxy-2,6-dimethylbenzene:
[0130] Sodium nitrite (34g, 0.50mol) was dissolved in 100mL of water under an ice bath, and 200g of crushed ice was dropped into it, and 100mL of sodium metabisulfate (Na 2 S 2 o 5 ) in aqueous solution (35%, w / v), and then add 20 mL of glacial acetic acid. After 3 minutes, 25 mL of concentrated ammonia (spgr0.8) was added. After reacting for 30 minutes, slowly add 400 mL of an aqueous solution of potassium permanganate (12.6 g, 0.079 mol) dropwise, continue stirring for 1 hour and filter, and pour the obtained purple filtrate into 800 mL of a saturated KCl solution under stirring. After stirring for 1 hour, filter to obtain Fermi's salt (Fremy's salt). The freshly prepared Fermi salt was dissolved in water at 0°C, and 400 mL of a methanol solution of 2,6-dimethylphenol (4.5 g, 0.037 mol) was slowly added dropwise with stirring. After reacting at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com