Aldehydic acids tetramethylpyrazine ester and method for preparing same
A technology of ligustrazine esters and polyacids, applied in the field of polyacids ligustrazine esters and its preparation, can solve the problems of fast excretion and low bioavailability of ligustrazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
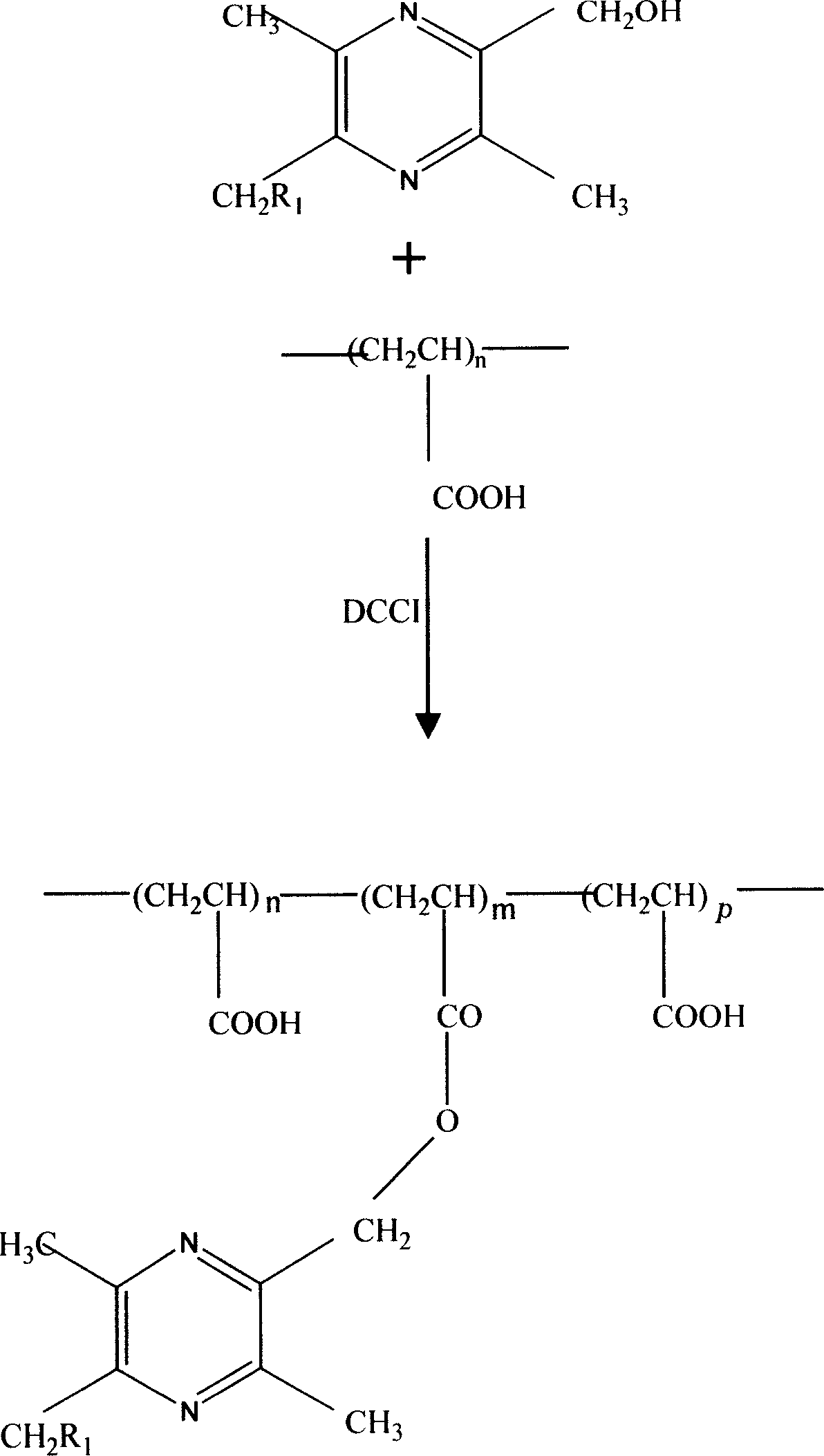
[0015] Synthesis of Ligustrazine Polyacrylate from Polyacrylic Acid with Molecular Weight of 800-1000
[0016] (1) Synthesis of 2,5-dimethylol-3,6-dimethylpyrazine (intermediate)
[0017] Dissolve 50.4g of ligustrazine phosphate in 90ml of glacial acetic acid, add 90ml of hydrogen peroxide solution in an oil bath at 98°C, reflux for 24 hours, adjust the pH value to 9-10 with 20% sodium hydroxide solution, and extract with chloroform. Add anhydrous Na 2 SO 4 Drying, filtering, and rotary evaporation can give white Ligustrazine-1,4-bis-nitrogen oxide with a melting point of 212-224°C. Weigh 16.8g ligustrazine-1,4-dinitrogen oxide and dissolve it in 150ml acetic anhydride, reflux for 4h under the condition of oil bath at 150°C, adjust the pH value to 9-10 with 20% sodium hydroxide solution, and let stand 24 hours, extracted with ethyl acetate, added anhydrous Na 2 SO 4 After drying, filtering and rotary evaporation, light yellow 2,5-dimethylol-3,6-dimethylpyrazine can be obt...
preparation example 2
[0023] The steps of Preparation Example 1 were repeated, and polyacrylic acid with a molecular weight of 100,000 was used instead of polyacrylic acid with a molecular weight of 800-1000 to prepare 2,4-dimethylol-3,6-dimethylpyrazine grafted polypropylene ester.
preparation example 3
[0025] Weigh 699.2 mg of 2-hydroxymethyl-3,5,6-trimethylpyrazine to replace 2,5-dimethylol-3,6-dimethylpyrazine, and repeat the steps of Preparation Example 1. Wherein the synthesis of 2-hydroxymethyl-3,5,6-trimethylpyrazine refers to Chinese patent (application number 200310100267X).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com