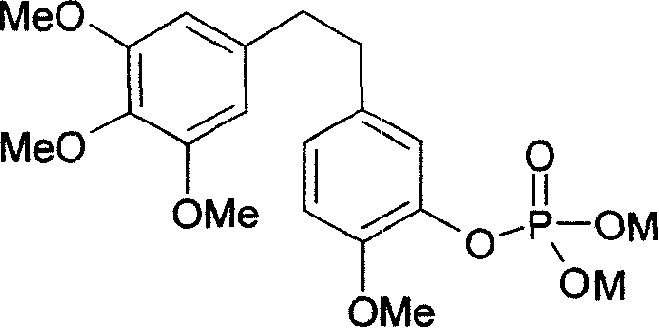
3-hydroxy-4,3',4',5'-tetromethoxy bibenzyl phosphate and its composition, prepn and application
A technology of methoxyphenyl and p-methoxybenzene, applied in the direction of phosphorus organic compounds, etc., can solve problems such as poor water solubility, and achieve the effects of less environmental pollution, low reaction cost, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
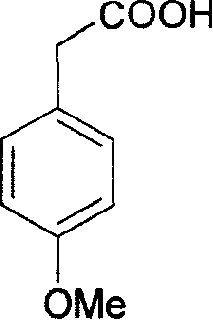
[0044] Take 10.2g (0.061mol) of p-methoxyphenylacetic acid in a three-necked flask, add 20mL of glacial acetic acid to dissolve it, then add 3.6mL of bromine (11.2g, 0.068mol) dropwise, drop it for 45 minutes, and then stir in an ice bath 1 hour, poured into ice water, precipitated solid, filtered, and dried to obtain 14.8 g of 3-bromo-4-methoxy-phenylacetic acid, the yield was 98.3% (the literature value was 98%), and the mixture was weighed with ethanol-water White flaky crystals were obtained by crystallization with a yield of 70% and a melting point of 113-114°C (literature value of 113-114°C).
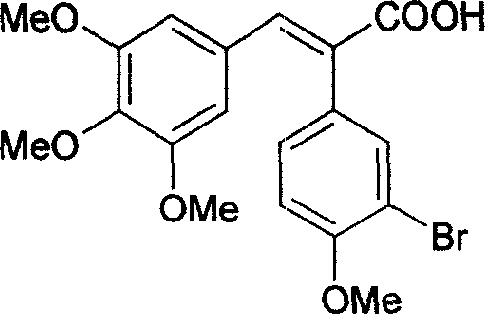
[0045]Weigh 4.92g (0.02mol) of the obtained 3-bromo-4-methoxyphenylacetic acid, and add 4.32g (0.022mol) of 3,4,5-trimethoxybenzaldehyde into the reaction flask, and use 20mL Acetic anhydride was dissolved, and 5.0 mL of triethylamine was added dropwise, heated to 130°C, and reacted for 5 hours. After acidification with concentrated hydrochloric acid, it was poured into ice water...
Embodiment 2
[0050] Embodiment 2: Inhibitory test to mouse P388 leukemia cells
[0051] Press 10 -6 mol / L 3-hydroxyl-4,3',4',5'-tetramethoxybibenzyl phosphate disodium salt prepared in Example 1 was applied to mouse P388 leukemia cells for 72 hours, and the inhibition of tumor cells The rate reaches 75% to 90%.
Embodiment 3
[0052] Embodiment 3: To the human HL-60 leukemia cell inhibition test
[0053] Press 10 -8 mol / L 3-hydroxyl-4,3 ', 4', 5'-tetramethoxybibenzyl phosphate disodium salt prepared in Example 1 was applied to human HL-60 leukemia cells for 72 hours, and the effect on tumor cells The inhibition rate is between 99% and 100%.
[0054] Embodiment 2~3 is the test that we entrust National Center for New Drug Screening to do. The test results of Examples 2-3 show that 3-hydroxy-4,3',4',5'-tetramethoxybibenzyl phosphate has very strong antitumor activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com