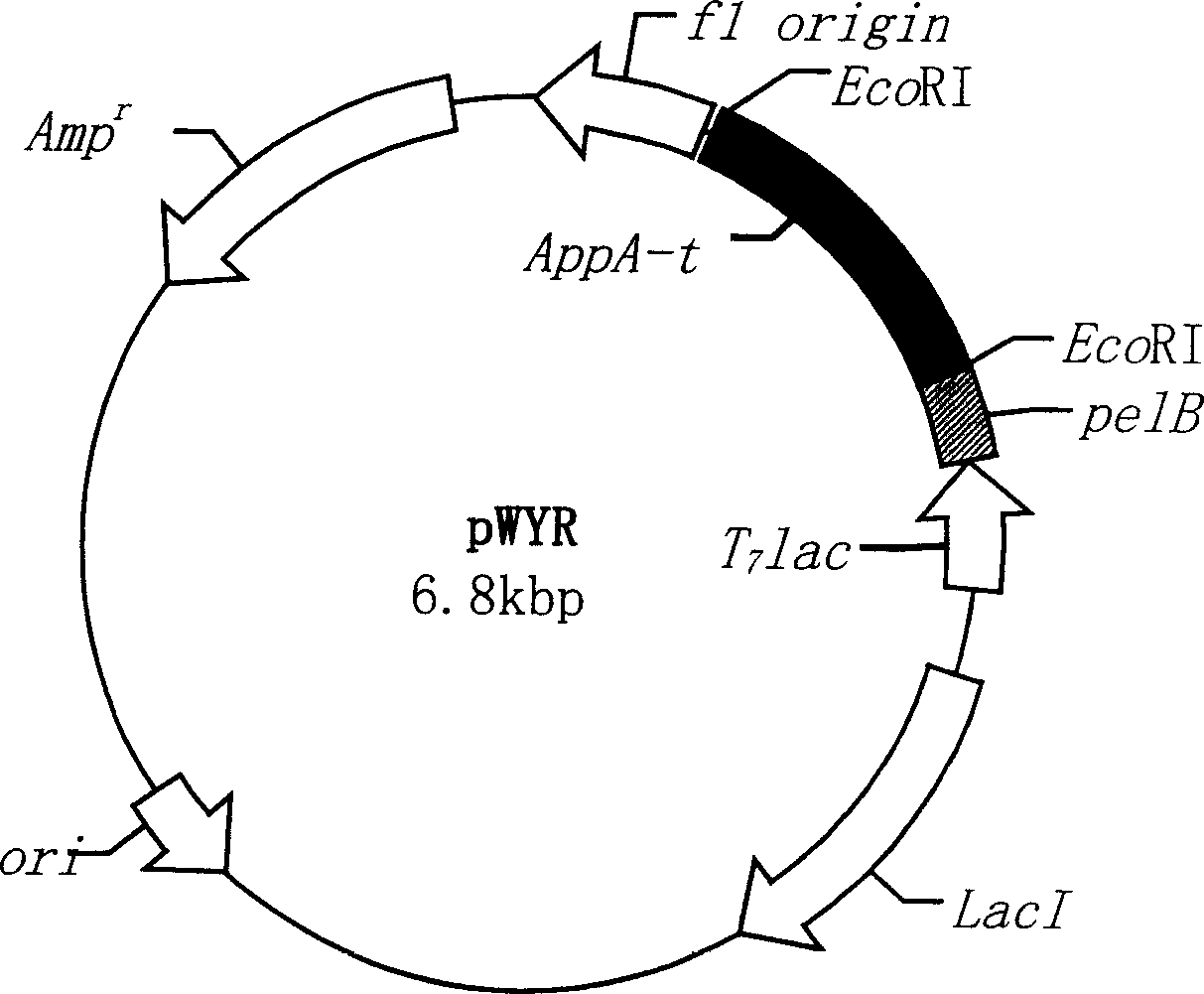
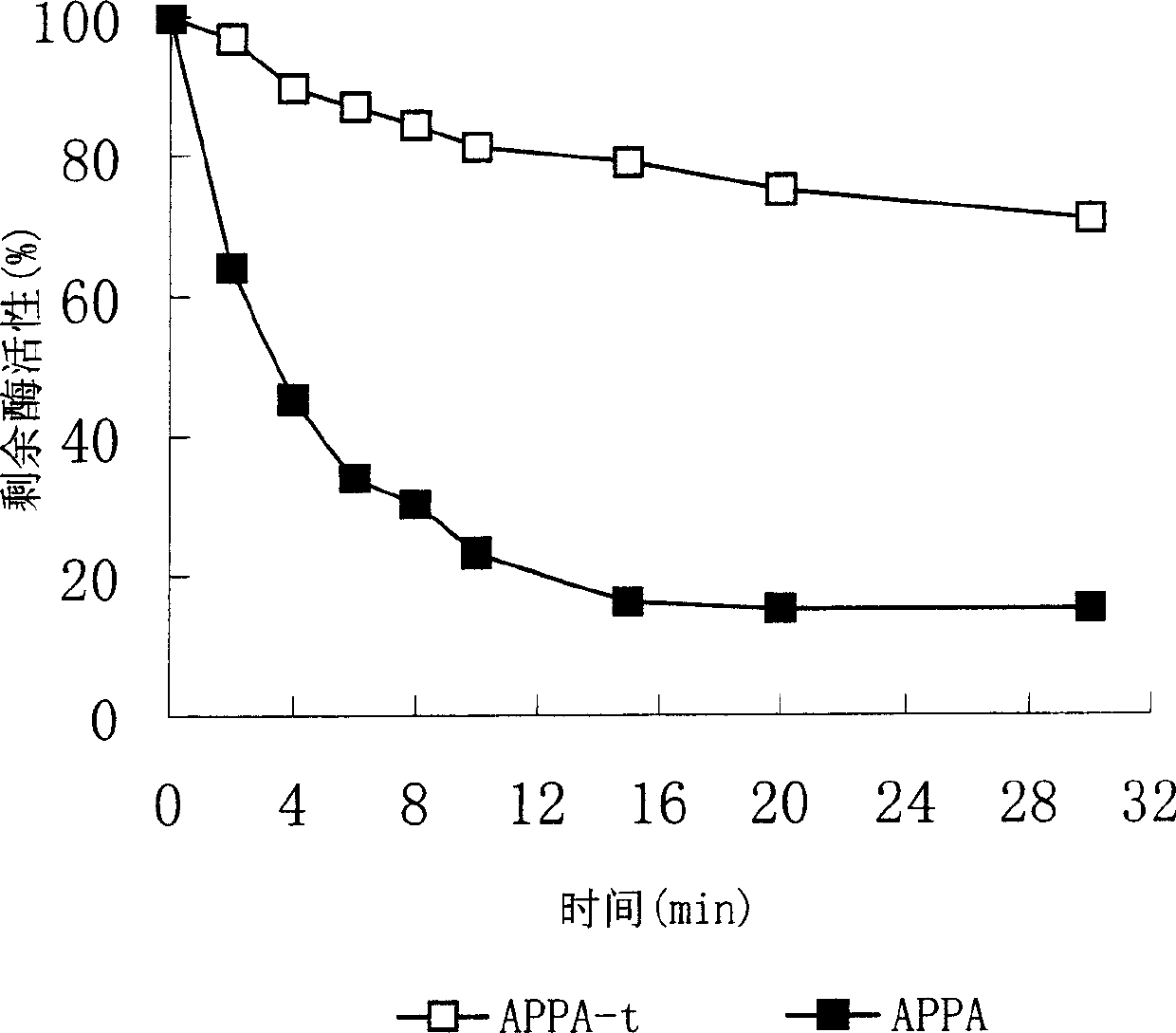
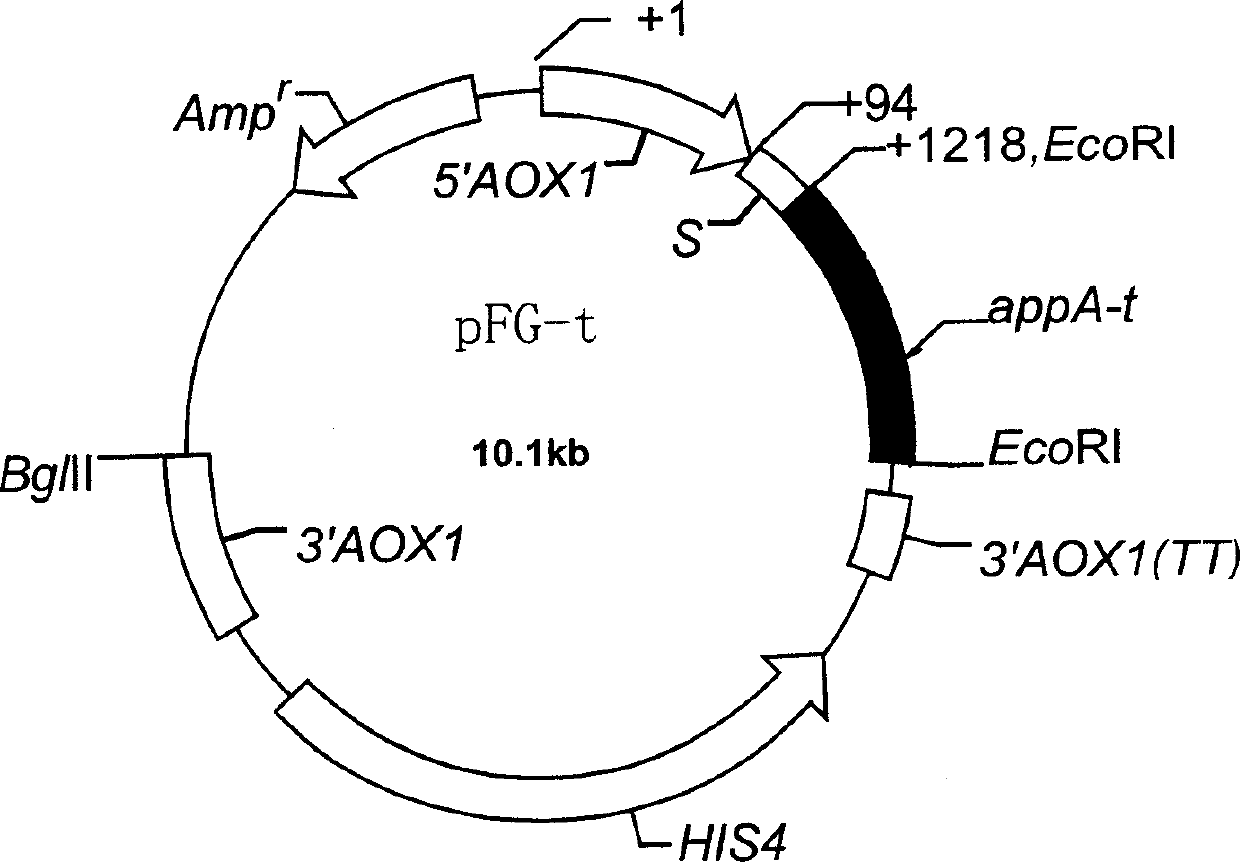
Heat stability improvement and efficient expression of phytase with high specific activity
A high-efficiency expression and thermal stability technology, applied in the field of improved phytase and its encoding gene, can solve the problems of poor thermal stability and large loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Experimental conditions
[0025] 1. Strains and vectors Escherichia coli strain E.coli DH5a and plasmid pET-22b(+) were purchased from Promega, and yeast strain Pichia pastoris GS115(His-Mut+) and plasmid pFF01 were kindly donated by Dr. D.Luo, University of Alberta, Canada.
[0026] 2. Enzymes and kits Restriction enzymes, ligases, Taq enzymes, and DNAaseI are products of Boehringer. PCR Kits were purchased from Promega.
[0027] 3. Biochemical reagents DNA synthesis reagents are products of Milipore Company. Primers were synthesized with ABI Cyclone DNA synthesizer. IPTG, X-Gal, SDS and sodium phytate are products of Sigma. TEMED, ammonium persulfate, acrylamide and methylene bisacrylamide are products of Promega.
[0028]4. Medium The culture medium for Escherichia coli is LB (1% peptone, 0.5% yeast extract, 1% NaCl, pH 7.0). Yeast complete medium is YPD (1% yeast extract, 2% peptone, 2% glucose); yeast transformation medium is RDB [18.6% sorbitol, 2% glucose, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com