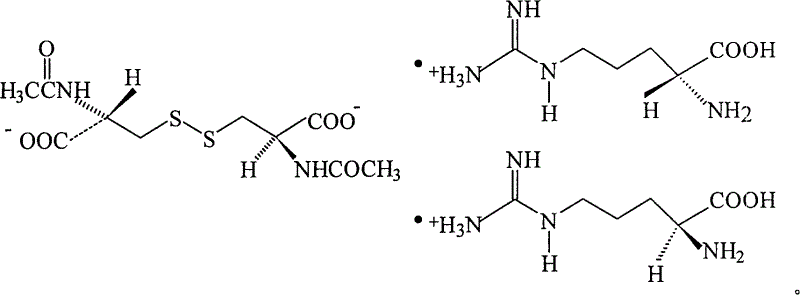
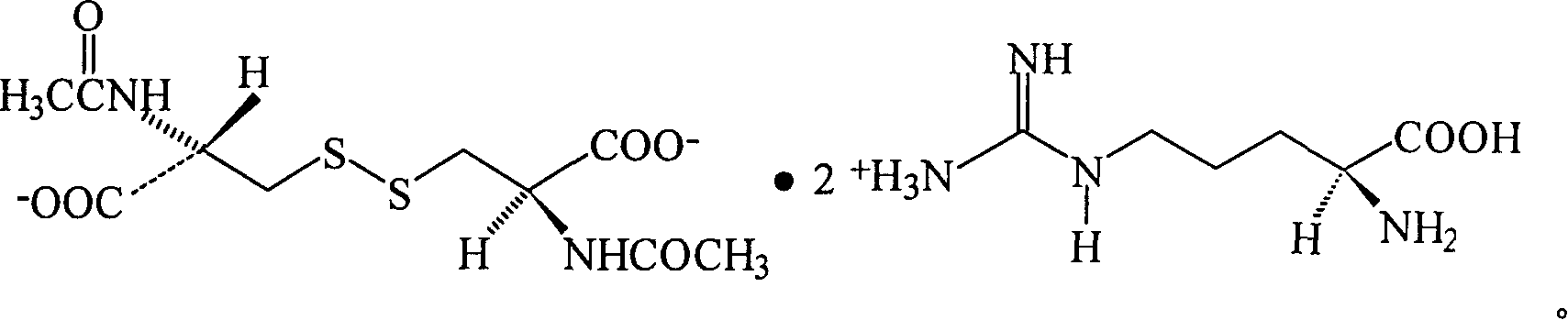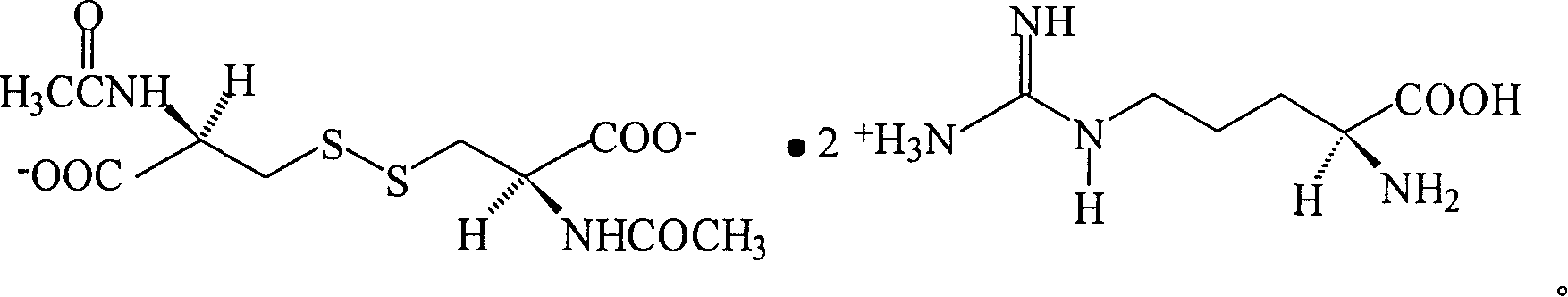N,N'-biacetylcysteine-diarginine salt isomer and its uses
A technology of diacetylcystine diarginine salt and isomers, which is applied in the field of arginine salts and can solve problems such as enhancing the activity of glutathione S transferase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 N, N'-diacetyl-L-cystine-L, the synthesis of L-diarginine salt
[0049] Put 672g of potassium hydroxide into a 5L reaction bottle, add 2400ml of water to dissolve it into concentrated lye, when the temperature drops to room temperature (20-30°C), add 360g of L-cystine, stir and dissolve, and cool to below 0°C. Slowly add acetic anhydride dropwise under cooling with an external ice-water bath, and control the internal temperature not to exceed 5°C. After the addition, continue to insulate and stir for 30 minutes, and place it for 3 hours. Add concentrated hydrochloric acid dropwise to adjust the pH to 2-3. Aqueous solution of acetylcystine.
[0050] The aqueous solution of acetylcystine was distilled under reduced pressure (external temperature 80°C), concentrated to a viscous state, extracted and filtered three times with acetone-water (90:10) to remove most of the inorganic salts. The extract was distilled off under reduced pressure to remove acetone, conc...
Embodiment 2
[0058] Example 2 N, N'-diacetyl-D-cystine-L, the synthesis of L-diarginine salt
[0059] Put 670g of potassium hydroxide into a 5L reaction bottle, add 2400ml of water to dissolve it into a concentrated lye, and when it cools down to room temperature (20-30°C), add 360g of D-cystine in batches, stir and dissolve, and cool to below 0°C. Slowly add acetic anhydride dropwise under cooling with an external ice-water bath, and control the internal temperature not to exceed 5°C. After the addition, continue to insulate and stir for 30 minutes, and place it for 3 hours. Add concentrated hydrochloric acid dropwise to adjust the pH to 2-3. Aqueous solution of acetylcystine.
[0060] The aqueous solution of acetylcystine was distilled under reduced pressure (external temperature 80°C), concentrated to a viscous state, extracted and filtered three times with acetone-water (90:10) to remove most of the inorganic salts. The extract was distilled off under reduced pressure to remove aceton...
Embodiment 3
[0063] Example 3 N, N'-diacetyl-L-cystine-D, the synthesis of D-diarginine salt
[0064] Put 675g of potassium hydroxide into a 5L reaction bottle, add 2400ml of water to dissolve it into a concentrated lye, and when it cools down to room temperature (20-30°C), add 360g of L-cystine, stir and dissolve, and cool to below 0°C. Slowly add acetic anhydride dropwise under cooling with an external ice-water bath, and control the internal temperature not to exceed 5°C. After the addition, continue to insulate and stir for 30 minutes, and place it for 3 hours. Add concentrated hydrochloric acid dropwise to adjust the pH to 2-3. Aqueous solution of acetylcystine.
[0065] The aqueous solution of acetylcystine was distilled under reduced pressure (external temperature 80°C), concentrated to a viscous state, extracted and filtered three times with acetone-water (90:10) to remove most of the inorganic salts. The extract was distilled off under reduced pressure to remove acetone, concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com