Single domain antibodies directed against tumor necrosis factor alpha and uses therefor
A single-domain antibody, tumor necrosis factor technology, applied in the direction of antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, application, etc., can solve the problem of protease degradation sensitivity, resistance, instability and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0290] Example 1: Examples of camelid antibodies against human tumor necrosis factor alpha
[0291] 1) Immunization and library construction
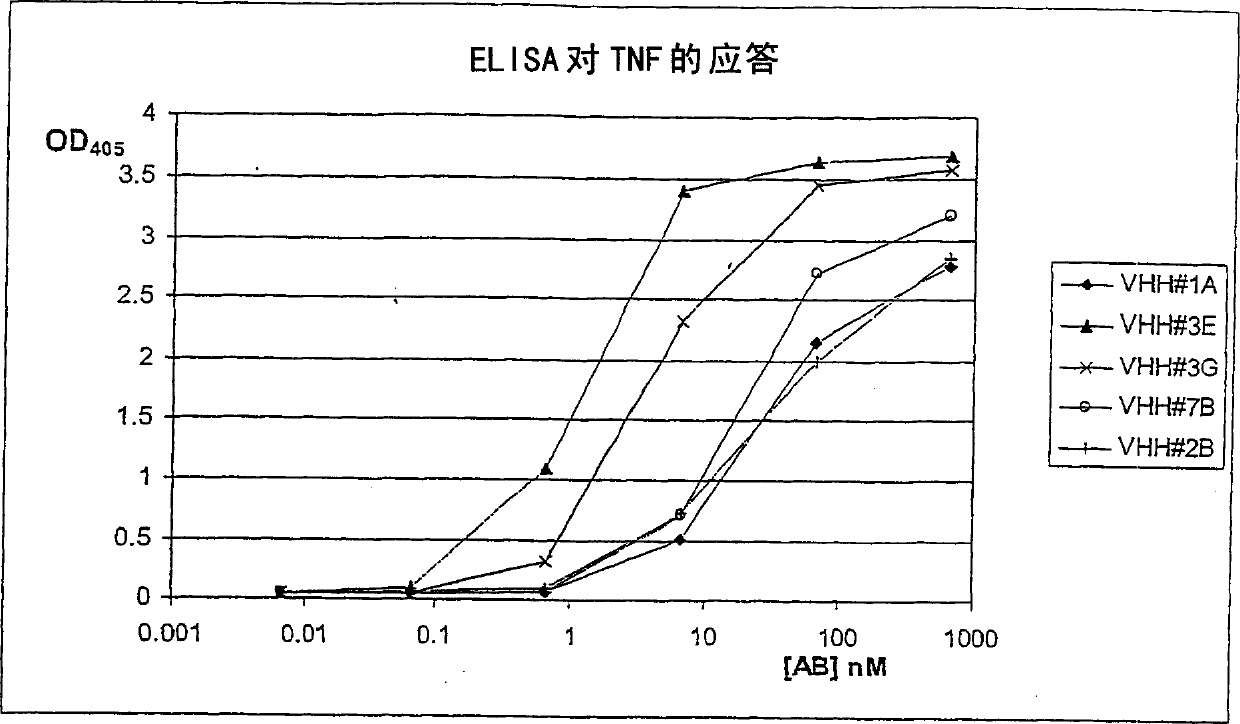
[0292] Llama (Llama glama) was immunized with human TNF-[alpha]. For immunization, cytokines are formulated as emulsions with appropriate animal-friendly adjuvants (Specoll, CEDI Diagnostics B.V.). The antigen mixture was administered by intramuscular dual site injection in the neck. Animals received 6 injections of emulsion containing 100 μg TNF-[alpha] at weekly intervals. At various time points during the immunization, 10 ml blood samples were collected from the animals and serum was prepared. The induction of an antigen-specific humoral immune response was verified with TNF in an ELISA experiment using serum samples (data not shown). 5 days after the last immunization, a 150 ml blood sample was collected. Conventional and heavy chain antibodies (HcAbs) were fractionated (Lauwereys et al., 1998) from this sample and used in ELIS...
Embodiment 2
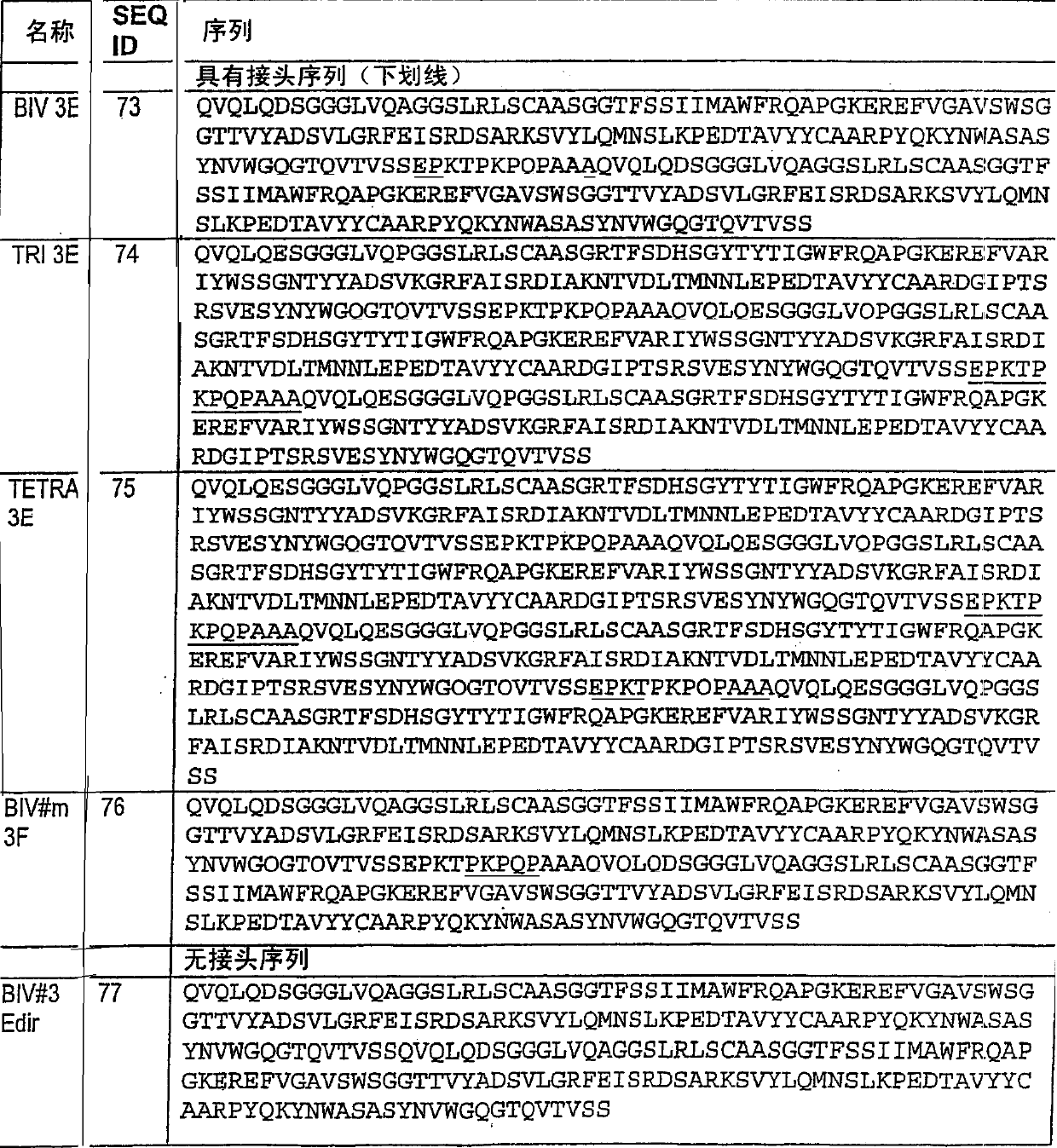
[0306] Example 2: Humanization of VHH#12B and VHH#3E by site-directed mutagenesis
[0307] 1) Homology between VHH#3E / VHH#12B and human germline heavy chain V-region DP-47 Alignment of VHH#12B and human VH3 germline sequence (DP-47) revealed a high degree of homology :
[0308] 4 amino acid changes at positions 1, 5, 28 and 30 in FR1
[0309] 5 amino acid changes at positions 74, 76, 83, 84 and 93 of FR3
[0310] 1 amino acid change at position 108 in FR4
[0311] As represented in the sequence alignment below:
[0312] DP-47 EVQLLESGGGLVQPGGSLRLSCAASGFTFS SYAMS WVRQAPGKGLEWVS AISGSGGSTYY
[0313] VHH#12B QVQLQESGGGLVQPGGSLRLSCAASGFEFE NHWMY WVRQAPGKGLEWVS TVNTNGLITRY
[0314] DP-47 ADSVKG RFTISRDNSKNTLYLQMNSLRAEDTAVYYCAK -------------- -----------
[0315] VHH#12B ADSVKG RFTISRDNAKYTLYLQMNSLKSEDTAVYYCTK VLPPYSDDSRTNAD WGQGTQVTVSS
[0316] Therefore, specific inhibitors of TNF-α cytokines with high homology to the human germline gene DP-47 are ideal c...
Embodiment 3
[0333] Example 3: Isolation of antagonistic VHH against mouse TNF-α
[0334] 1) Select anti-mouse TNF-α VHH
[0335] For efficacy studies in IBD or Crohn's disease mouse models, mouse TNF-specific VHHs were selected. Therefore, llamas were immunized with mouse TNF-[alpha] as described in Example 1. RNA was extracted from PBLs sampled 4 and 10 days after the last immunization and from lymph node biopsies after the fourth day. Total RNA was converted to random-primed or oligo-dT-primed cDNA and used as template for amplification of VHH-encoding gene segments using Ig-derived primers or a mixture of oligo-dT primers and a single Ig primer (see Example 1). From the first PBL's generated using Ig primers containing 8.5 x 10 7 A library of libraries of clones was generated from a second PBL sample of 7 x 10 6 A library of clones yielded 5.8 × 10 8 library of clones. A mixture of oligo-dT and Ig primers was obtained from the first PBL sample containing 1.2 × 10 8 A library of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com