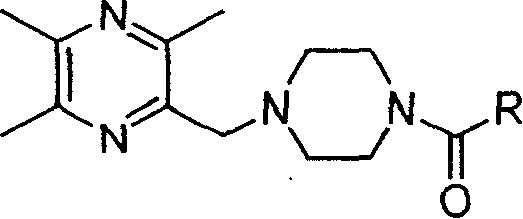
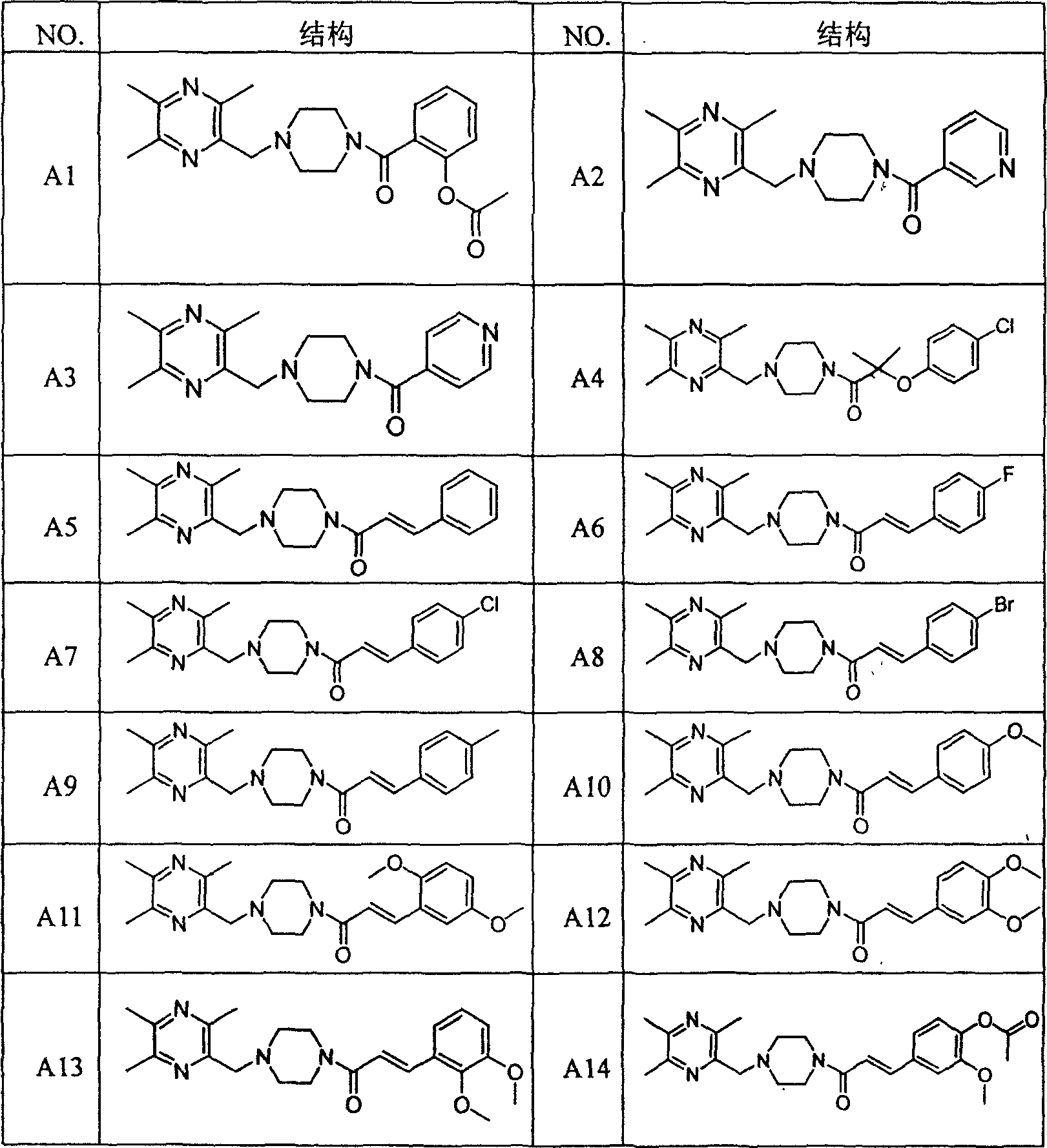
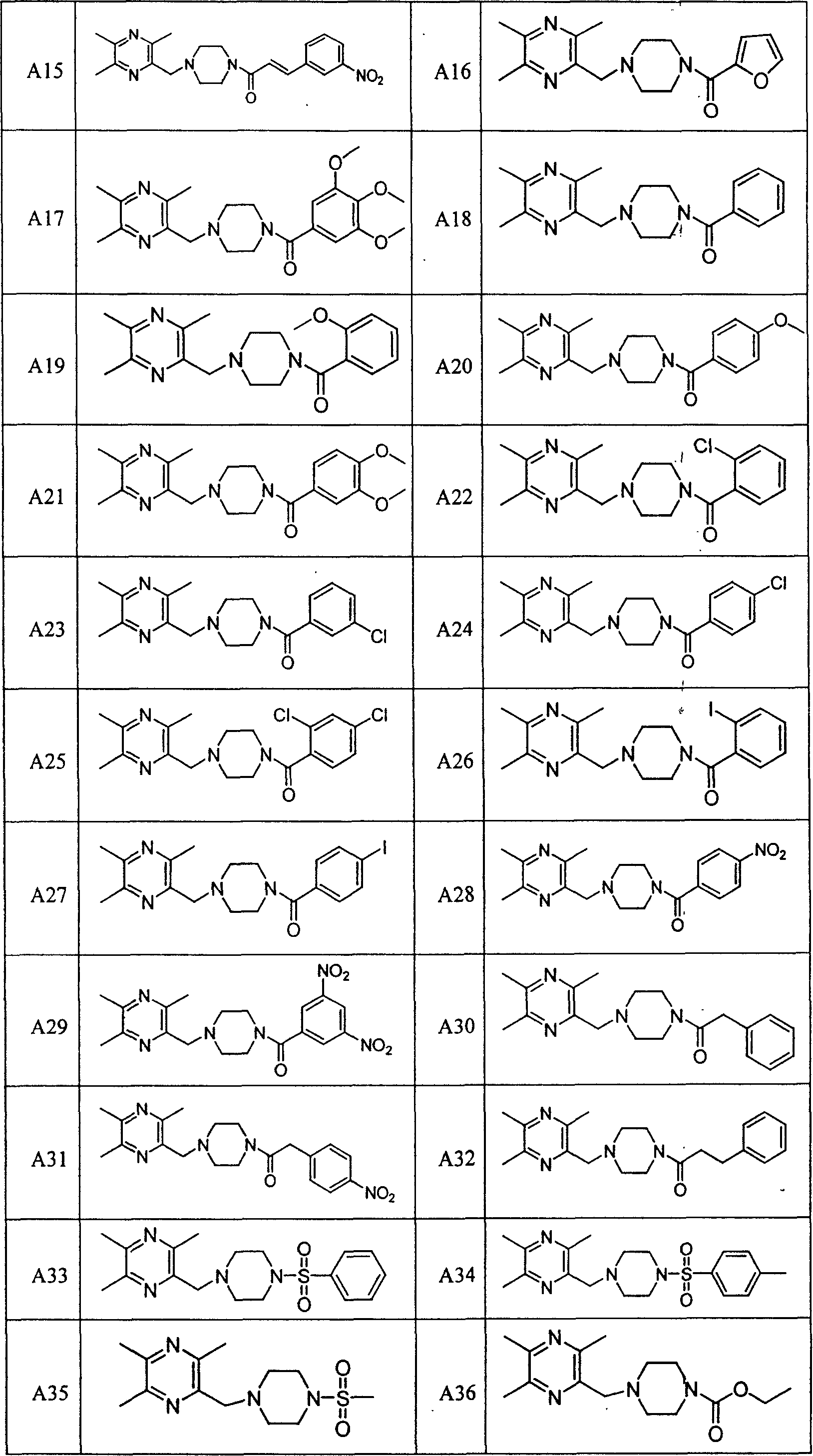
Liyustrazine acyl piperazine derivative, and its preparing method and medicinal composition and use
A technology of ligustrazinylpiperazine and derivatives, which is applied in the field of Ligusticum derivatives medicines, and can solve the problems of large toxic side effects and poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1. the preparation of intermediate 2-chloromethyl-3,5,6-trimethylpyrazine hydrochloride (2)
[0063] A mixture of Ligustrazine trihydrate (30.4g, 160mmol), glacial acetic acid (40ml) and 30% hydrogen peroxide (18ml, 160mmol) was heated at 70°C for 4h, and 30% hydrogen peroxide (18ml, 160mmol) was added , continue to react for 4h, TLC monitors until the reaction is complete, cool to room temperature, adjust pH=10 with 50% sodium hydroxide solution, extract with chloroform, dry over anhydrous sodium sulfate, filter, evaporate chloroform to obtain ligustrazine mono Crude nitrogen oxides. Then add acetic anhydride (15.1ml, 160mmol), heat to reflux for 2.5h, monitor by TLC until the reaction is complete, evaporate excess acetic anhydride under reduced pressure to obtain black slurry ligustrazine acetylate, add 20% sodium hydroxide solution after cooling (155ml), left overnight, extracted with chloroform (150ml, 30ml×5 times), dried over anhydrous sodium sulfate, ...
Embodiment 2
[0065] Embodiment 2. Preparation of intermediate N-acylpiperazine (3)
[0066] Take carboxylic acid (100mmol) and thionyl chloride (200mmol) into a round-bottomed flask, install a condenser tube and a drying tube, heat and reflux for 5h, evaporate excess thionyl chloride, and carry out vacuum distillation on the residue to obtain the acid chloride derivative things.
[0067] Put piperazine hexahydrate (11.7g, 60mmol) in a round-bottomed flask, add glacial acetic acid (50ml), stir until dissolved, add the above-synthesized acid chloride (50mmol) dropwise at room temperature, after completion of the dropwise addition, stir for 2h, add to the reaction Add water (50ml) to the solution, then adjust the pH=12 with sodium hydroxide solution, place it under cooling, remove a small amount of bisacylated product by filtration, extract the filtrate 5 times with chloroform, combine the extracts, wash with water until neutral, and use Dry over sodium sulfate, evaporate chloroform to get a...
Embodiment 3
[0068] Example 3. Preparation of intermediate 2-(1-piperazinylmethyl)-3,5,6-trimethylpyrazine (4)
[0069] Anhydrous piperazine (50g, 580mmol) was dissolved in chloroform (300ml), and 2-chloromethyl-3,5,6-trimethylpyrazine hydrochloride ( 20.7g, 100mmol) of chloroform (100ml) solution, react at room temperature for 5h, TLC monitors that the reaction is complete, the reaction solution is washed with 4mol / L ammonia water (100ml×3 times), the organic layer is dried with anhydrous sodium sulfate, filtered, evaporated After removing the solvent, 19 g of crude black oil was obtained with a yield of 86%, which could be directly used in the synthesis of the final product without purification. A small amount of the crude product was recrystallized with n-hexane to obtain the white crystalline intermediate 2-(1-piperazinylmethyl)-3,5,6-trimethylpyrazine (4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com