Method for determining gingko lactone content in gingko extraction and medical preparation
A technology of ginkgolide and its determination method, which is applied in the field of quality inspection of ginkgolide and its pharmaceutical preparations, and can solve the problems of inaccurate data, high price and complexity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Determination of Ginkgolide Extract Content
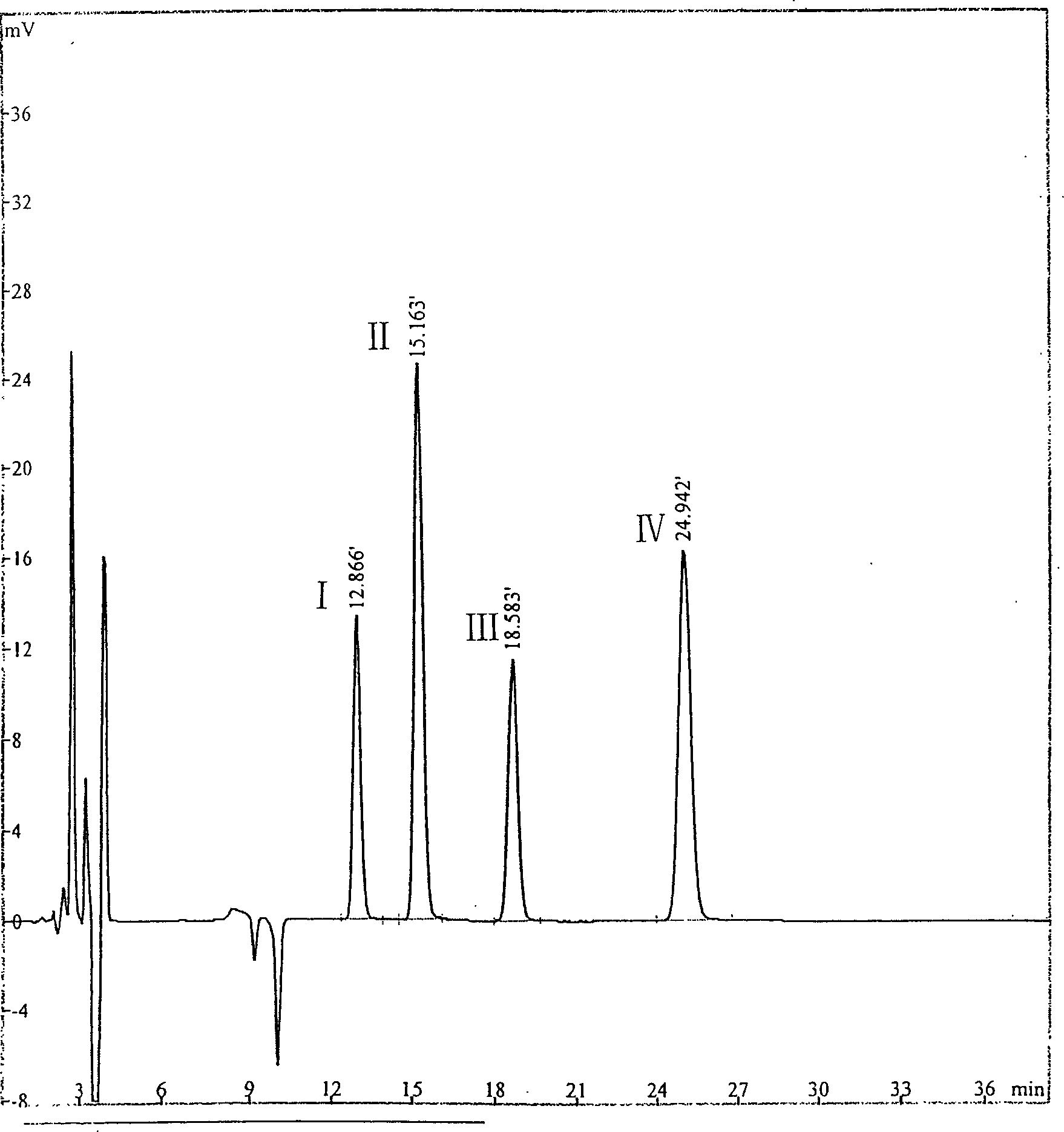
[0024] Chromatographic conditions: Kromasil's C18 column is used as a chromatographic column; methanol-tetrahydrofuran-water (25:10:65) is used as a mobile phase; the detection wavelength is 220nm; The separation degree should not be less than 1.5 based on ginkgolide C peak and bilobalide peak.
[0025] The preparation of need testing solution: precision takes ginkgolide appropriate amount, dissolves with mobile phase and makes the solution that every 1ml contains 5mg ginkgolide, as need testing solution;
[0026] Preparation of the reference solution: accurately weigh 18.12 mg of ginkgolide A, 8.31 mg of ginkgolide B, 10.48 mg of ginkgolide C and 19.46 mg of bilobalide, put them in a 10ml measuring bottle, add an appropriate amount of 50% methanol to dissolve and dilute To the scale, shake well to obtain a reference substance solution containing 1.81 mg of ginkgolide A, 0.83 mg of ginkgolide B, 1.05 mg of ginkgolide C, and...
example 2
[0029] Determination of Ginkgolide Injection
[0030] Chromatographic conditions: Kromasil's C18 column is used as a chromatographic column; methanol-tetrahydrofuran-water (25:10:65) is used as a mobile phase; the detection wavelength is 220nm; The separation degree should not be less than 1.5 based on ginkgolide C peak and bilobalide peak.
[0031] Need testing solution must be prepared: ginkgolide injection specification is 10mg / 2ml, precision measures this injection 1ml, as need testing solution;
[0032] Preparation of reference solution: Accurately weigh 8.80 mg of ginkgolide A, 4.15 mg of ginkgolide B, 4.90 mg of ginkgolide C and 10.05 mg of bilobalide, put them in a 5ml measuring bottle, add appropriate amount of methanol to dissolve and dilute with mobile phase to the scale, shake well, and obtain a reference solution containing 1.76 mg of ginkgolide A, 0.83 mg of ginkgolide B, 0.98 mg of ginkgolide C, and 2.01 mg of bilobalide per ml;
[0033] Determination method: ...
example 3
[0035] Determination of Ginkgolide for Injection
[0036] Chromatographic conditions: Kromasil's C18 column is used as a chromatographic column; methanol-tetrahydrofuran-water (25:10:65) is used as a mobile phase; the detection wavelength is 220nm; The separation degree should not be less than 1.5 according to the ginkgolide C peak and the bilobalide peak.
[0037] Preparation of the test solution: Accurately weigh an appropriate amount of content under the average loading of ginkgolide for injection, dissolve it with mobile phase and make a ginkgolide solution containing 5 mg per 1 ml, as the test solution;
[0038] Preparation of the reference solution: accurately weigh ginkgolide A18.37mg, ginkgolide B8.14mg, ginkgolide C10.32mg and bilobalide 20.48mg, put in a 10ml measuring bottle, add appropriate amount of 50% methanol to dissolve and dilute To the scale, shake well to obtain a reference solution containing 1.84 mg of ginkgolide A, 0.81 mg of ginkgolide B, 1.03 mg of gi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com