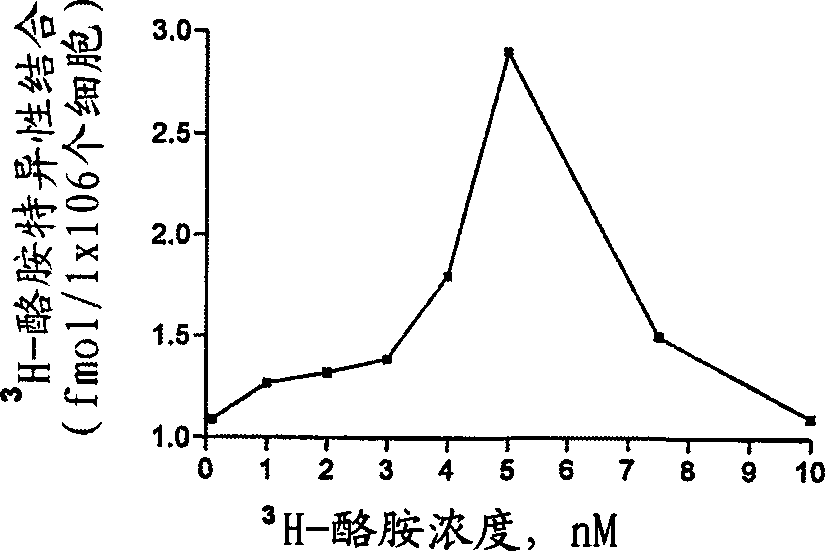
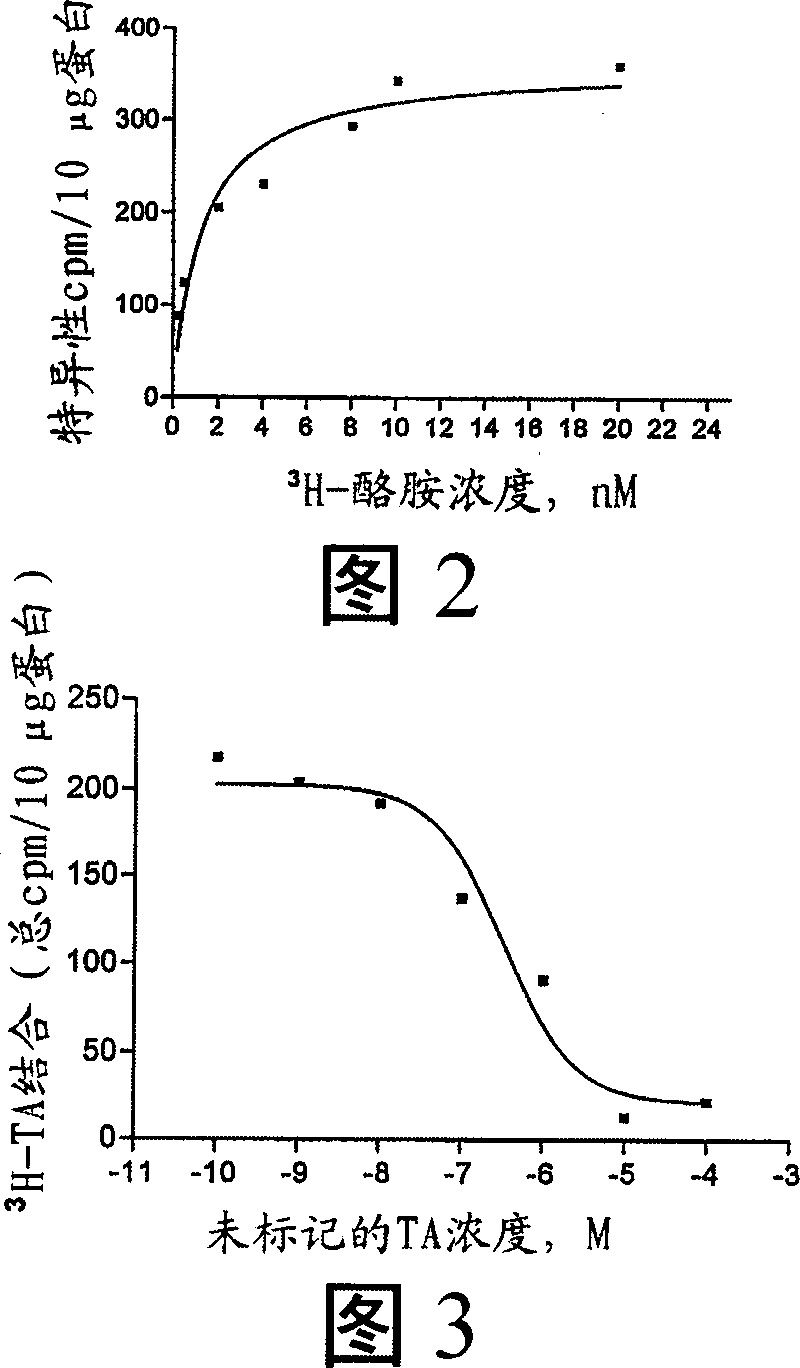
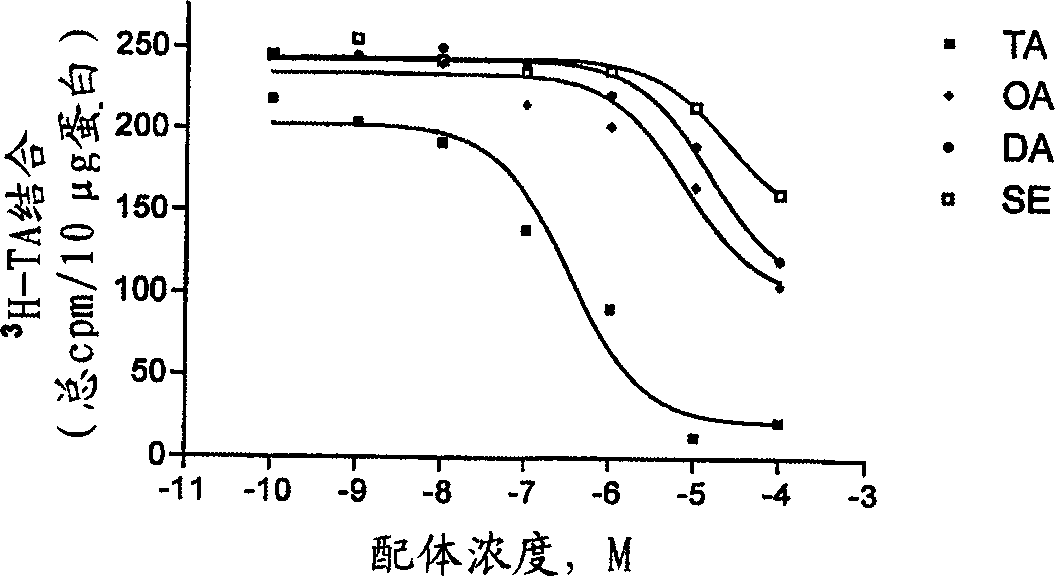
Compositions and methods for controlling insects
A composition and technology for insects, applied in the field of compositions and methods for controlling insects, can solve the problems of different insect functions, difficult identification of compounds, vertebrate heart poisoning, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Preparation of Schneider cell line stably transfected with tyramine receptor (TyrR)
[0121] A. PCR amplification and subcloning of Drosophila melanogaster tyramine receptors
[0122] Tyramine receptors were amplified from the head cDNA phage library GH of Drosophila melanogaster obtained from the Berkeley Drosophila Genome Project (Baumann, A., 1999, Drosophila melanogaster mRNA for octopamine receptor, splice variant 1B NCBI direct submission, AccessionAJ007617) . The nucleic acid sequence and peptide sequence of TyrR are as Figure 31A and 31B shown. Phage DNA was purified from liquid culture lysates of this library. (Baxter, et al., 1999, Insect Biochem Mol Biol 29, 461-467). Open reading frame for amplification of the Drosophila tyramine receptor (TyrR) (Han, et al., 1998, J Neurosci 18, 3650-3658; von Nickisch-Rosenegk, et al., 1996. Insect BiochemMol Biol 26, 817-827) is a 5' oligonucleotide: 5' gccgaattc gc caccATGCCATCGGCAGATCAGATCCTG 3' and 3' oligos...
Embodiment 2
[0140] Effects of Treatment of Cells Expressing Tyramine Receptors and Compositions on Intracellular [cAMP]
[0141] Cells were cultured on plates and the medium was changed the day before treatment. When the cells reached approximately 95% confluency, the culture medium was aspirated and replaced with 5ml insect saline (170mM NaCl, 6.0mM KCl, 2.0mM NaHCO 3 , 17.0mM Glucose, 6.0mM NaH 2 PO 4 , 2.0 mM CaCl 2 , 4.0 mM MgCl 2 ; pH 7.0) to wash the cells once. Add approximately 20ml of insect saline, and gently scrape to collect the cells. An aliquot of cells was counted with a hemocytometer, and the cells were centrifuged at 1000 RPM for approximately 5 minutes. Resuspend cells to 3 x 10 6 cells / ml. IBMX was added to a concentration of about 200 µM. Then about 1ml of the cell suspension was aliquoted for processing. Forskolin (cAMP inducing reagent), tyramine or different candidate combinations are added and the cells are incubated at about 27°C for about 10 minutes.
[...
Embodiment 3
[0145] Treatment of Cells Expressing Tyramine Receptors and Compositions for Intracellular [Ca 2+ ]Effect
[0146] The intracellular calcium ion concentration was determined by the fluorescent indicator furan-2 acetoxymethyl (AM) ester ([Ca 2+ ] i) (Enan, et al., Biochem. Pharmacol vol 51, 447-454). In this study, cells expressing tyramine were cultured under standard conditions. Zhou detection buffer (140mM NaCl, 10mM HEPES, 10mM glucose, 5mM KCl, 1mM CaCl 2 , 1 mM MgCl 2 ) to prepare cell suspension, adjust the number of cells to approximately 2×10 per ml 6 cells. Briefly, approximately 1.0ml of the suspension (approximately 2×10 6 cells) were incubated with 5 μM furan 2 / AM for about 30 minutes at about 28°C. After incubation, cells were pelleted by centrifugation at 3700 rpm for approximately 10 seconds at room temperature, and then resuspended in approximately 1.5 ml of assay buffer. [Ca 2+ ]i changes. The excitation wavelength is about 340nm (by binding Ca 2+ p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com