Hydrotalcite with photochromic characteristic and its prepn process
A photochromic and hydrotalcite technology, applied in the field of hydrotalcite, achieves the effects of pollution-free preparation, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Step A: 21.76g (0.1mol) of solid Ni(NO 3 ) 2 ·6H 2 O and 14g (0.05mol) of solid Al(NO 3 ) 3 9H 2 O was dissolved in 100ml of water.
[0037] Step B: Dissolve 12g NaOH in 100ml water to prepare a 3.0M alkaline solution.
[0038] Step C: While vigorously stirring, slowly add the alkali solution to the salt solution dropwise, adjust the pH=6 to complete the dropwise addition, crystallize at 70°C for 24h, filter and wash until the pH value is about 7, and dry at 70°C for 24h to obtain nickel Aluminum nitrate-type hydrotalcite material.
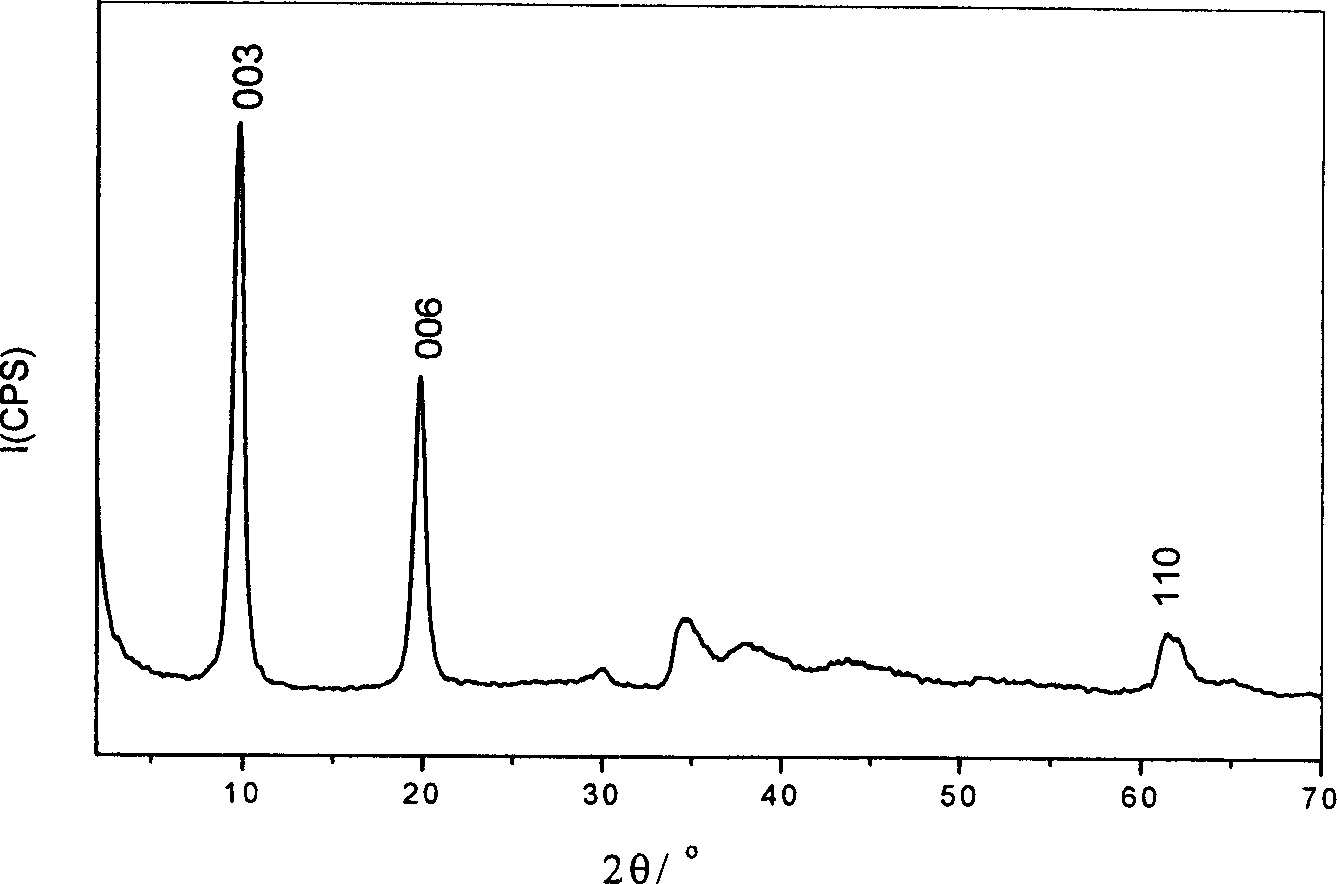
[0039] By XRD spectrum ( figure 1 ), IR spectrum and elemental analysis show that the interlayer anions of the obtained hydrotalcite are all nitrate, which is a LDHs material with a single crystal phase and a consistent structure. Its chemical formula / composition is: [Ni 0.643 al 0.357 (OH) 2 ](NO 3 - ) 0.357 0.7H 2 O.
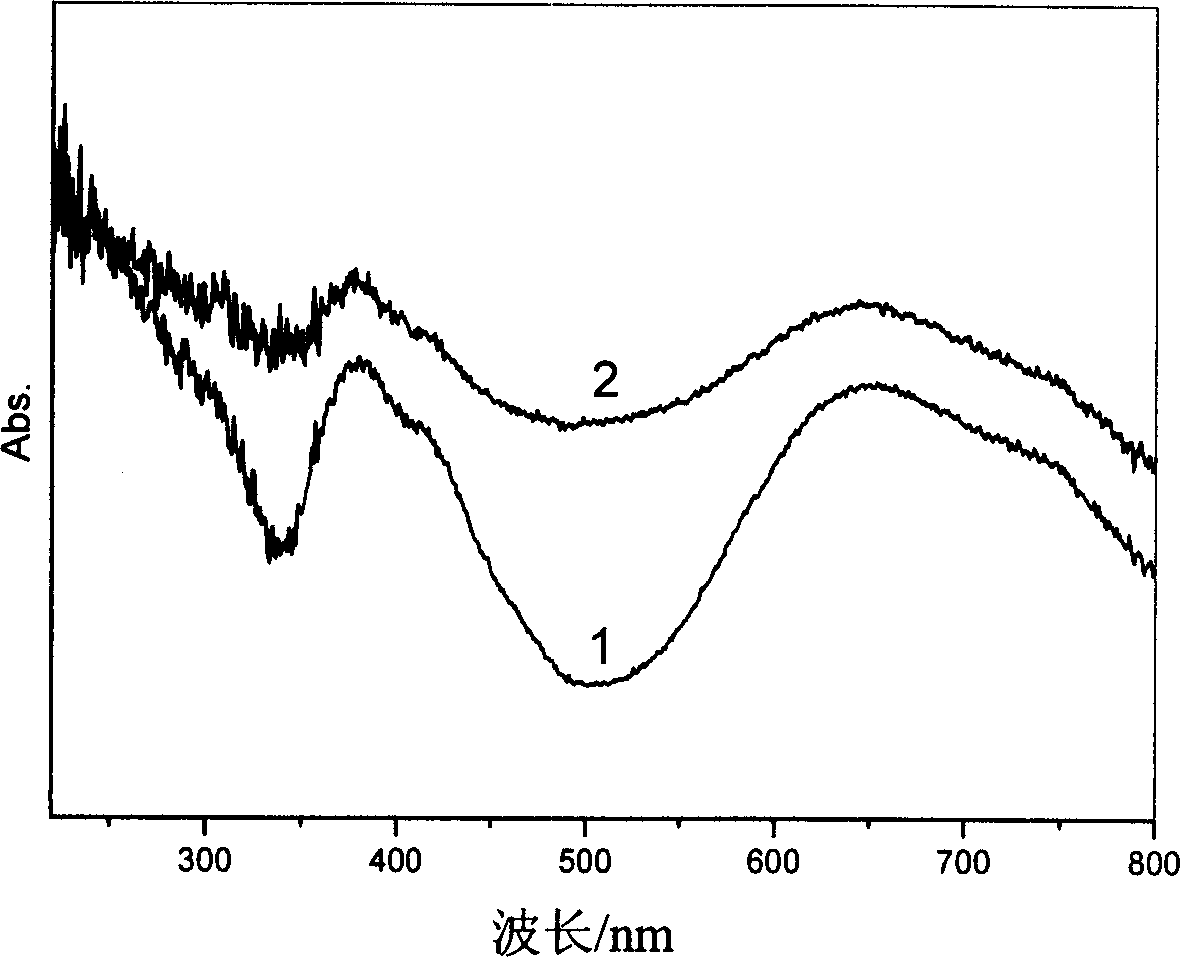
[0040] The LDHs was irradiated under a 500W high-pressure xenon lamp for 20 minutes, and its color change...
Embodiment 2
[0042] Step A: 3.56g NiCl2 ·6H 2 O and 1.81 g AlCl 3 9H 2 O was dissolved in 25ml of water.
[0043] Step B: Dissolve a certain amount of p-nitrobenzoic acid (1.8g) and 6.2g NaOH in 100ml water to form a mixed alkali solution.
[0044] Step C: While stirring vigorously, slowly add the salt solution dropwise to the mixed alkali solution, adjust the pH=9 to complete the dropwise addition, crystallize at 70°C for 24h, filter, wash until the pH value is about 7, and dry at 70°C for 24h to obtain p-Nitrobenzoic acid intercalation nickel aluminum hydrotalcite material.
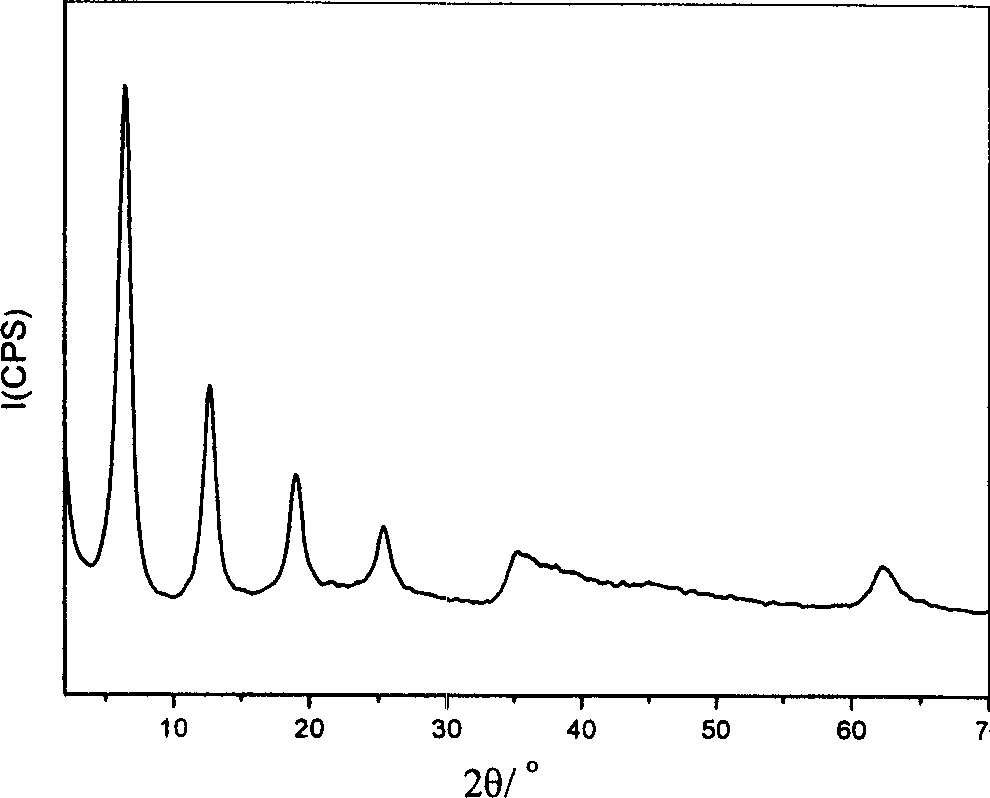
[0045] By XRD spectrum ( image 3 ), IR spectrum and elemental analysis, it can be seen that the interlayer anions of the obtained hydrotalcite are all p-nitrobenzoate, which is a LDHs material with a single crystal phase and a consistent structure. Its chemical formula / composition is: [Ni 0.655 al 0.345 (OH) 2 ](C 7 h 4 NO 4 - ) 0.345 0.7H 2 O.
[0046] The LDHs was irradiated under a 500W high-press...
Embodiment 3
[0048] Step A: 3.56g NiCl 2 ·6H 2 O and 1.81 g AlCl 3 9H 2 O was dissolved in 25ml of water.
[0049] Step B: Dissolve a certain amount of m-nitrobenzoic acid (1.8g) and 6.2g NaOH in 100ml water to form a mixed alkali solution.
[0050] Step C: While stirring vigorously, slowly add the salt solution dropwise to the mixed alkali solution, adjust the pH=9 to complete the dropwise addition, crystallize at 70°C for 24h, filter, wash until the pH value is about 7, and dry at 70°C for 24h to obtain Intercalation of nickel-aluminum hydrotalcite materials with m-nitrobenzoic acid.
[0051] From the XRD spectrum, IR spectrum and elemental analysis, it can be seen that the interlayer anions of the obtained hydrotalcite are all m-nitrobenzoate, which is a LDHs material with a single crystal phase and a consistent structure. Its chemical formula / composition is: [Ni 0.671 al 0.329 (OH) 2 ](C 7 h 4 NO 4 - ) 0.329 0.8H 2 O.
[0052] The LDHs was irradiated under a 500W high-pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com