Carbon-metal composite material and process of preparing the same
A metal composite material, metal technology, applied in gold-organic compounds, chemical instruments and methods, fuel cells, etc., can solve the problems of non-uniform composition of composite materials, difficult to prepare particle specific surface area, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] 3.8 g of nickel(II) acetate tetrahydrate and 2.0 g of trimesic acid were added to 100 mL of distilled water and stirred at 55° C. for 2 hours. The powder produced in the solution was separated using a nylon filter, washed several times with distilled water, and then dried in an oven at 100° C. for 2 hours to obtain a crystalline coordination polymer.
[0082] The obtained crystalline coordination polymer was heat-treated in an Ar atmosphere at 900 °C for 1 hour to prepare a carbon-nickel composite material with the same shape and reduced volume as the untreated crystalline coordination polymer.
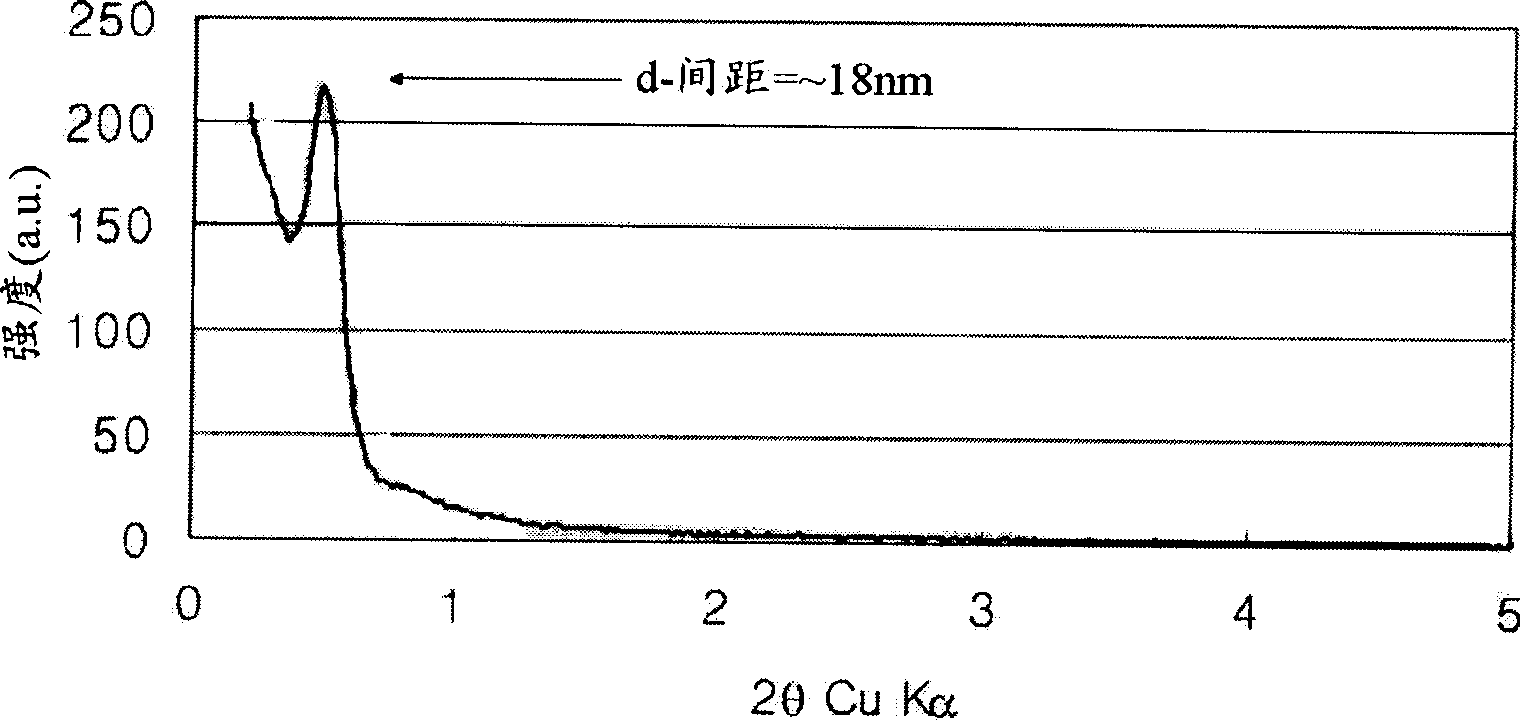
[0083] The carbon-nickel composite was tested using X-ray diffraction method. As a result, it was confirmed that the size of the nickel metal particles was 18.3 nm. refer to figure 1 , a periodicity of 18 nm can be observed when performing small-angle X-ray diffraction experiments.
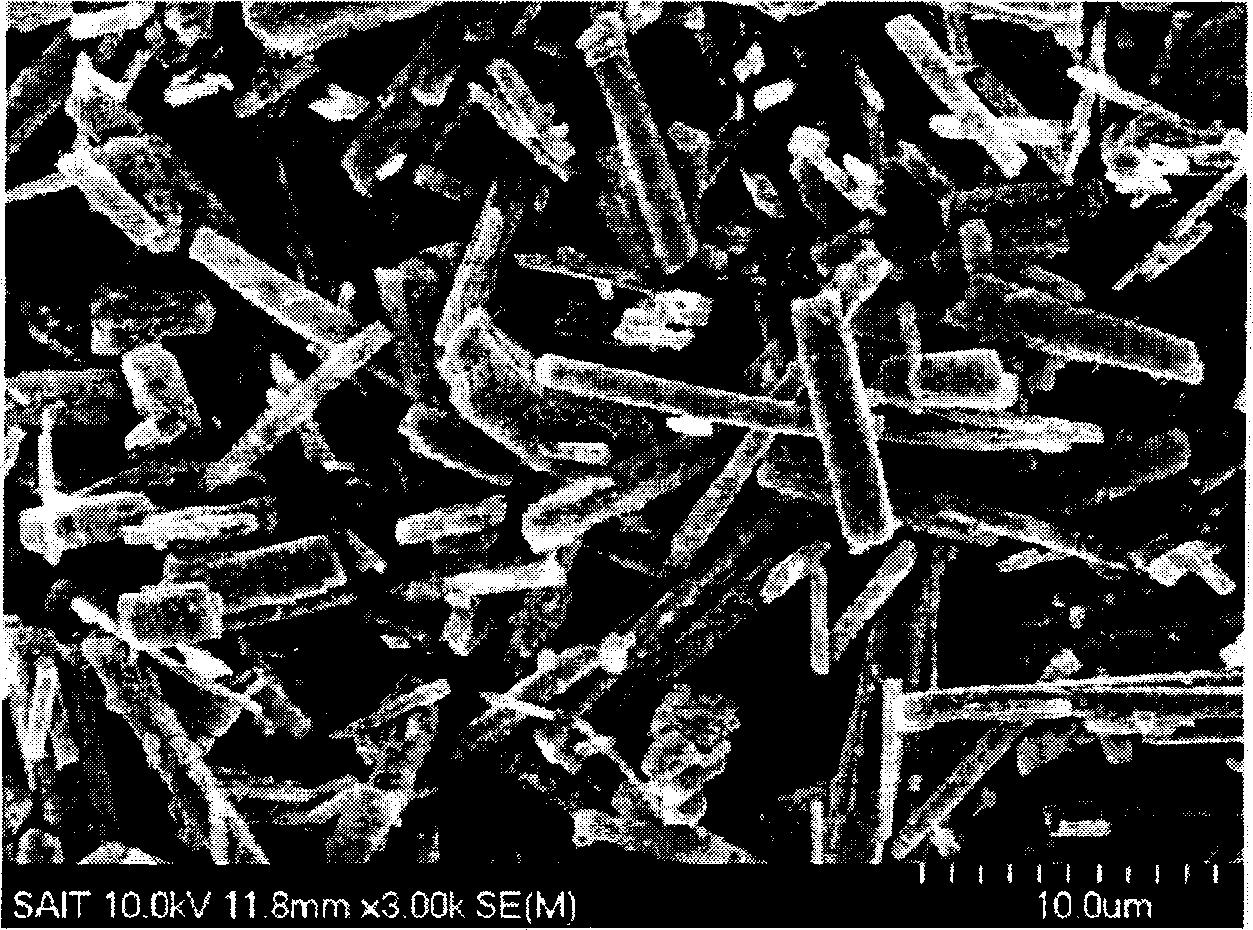
[0084] The SEM images of the untreated crystalline coordination polymer and the carbon-metal...
Embodiment 2
[0086] 3.8 g of nickel(II) acetate tetrahydrate and 2.0 g of trimesic acid were added to 100 mL of distilled water and stirred at room temperature for 2 hours. The powder produced in the solution was separated using a nylon filter, washed several times with distilled water, and then dried in an oven at 100° C. for 2 hours to obtain a crystalline coordination polymer.
[0087] The obtained crystalline coordination polymer was heat-treated in an Ar atmosphere at 900 °C for 1 hour to prepare a carbon-metal composite having the same shape and reduced volume as the untreated crystalline coordination polymer.
[0088] refer to Figure 4 , a periodicity of 29 nm can be observed when performing small-angle X-ray diffraction experiments.
[0089] The SEM images of the untreated crystalline coordination polymer and the carbon-metal composite obtained after heat treatment are in Figure 5 with 6 shown in . from Figure 5 with 6 It can be seen that although the density of the carbon...
Embodiment 3
[0091] A carbon-nickel composite material was prepared in the same manner as in Example 1, except that the synthesis temperature of the coordination polymer was changed from 55°C to 100°C.
[0092] The SEM images of the untreated crystalline coordination polymer and the carbon-metal composite obtained after heat treatment are in Figure 7 with 8 shown in . from Figure 7 with 8 It can be seen that although the density of the carbon-metal composite after heat treatment is higher than that before heat treatment due to the decrease in volume, the original crystal structure is maintained. Therefore, the carbon-metal composite has a regular shape.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com