In situ determination for polymer conformation and crystal-form variation in supercritical fluid infrared spectrum
A technology of supercritical fluid and infrared spectroscopy, which is used in the field of conformation and crystal form changes of high-molecular polymers, and can solve the problems of inability to obtain changes and trends.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
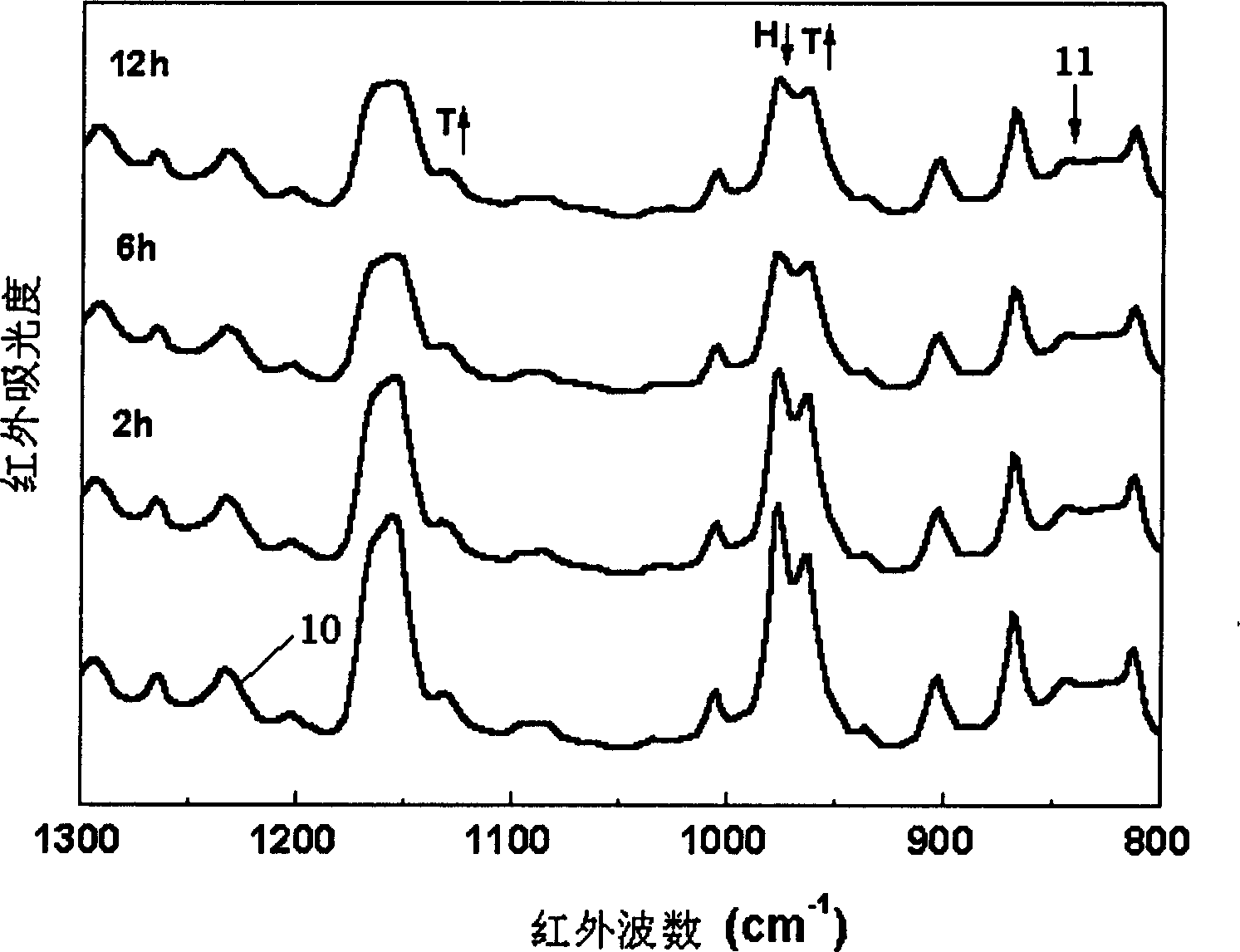
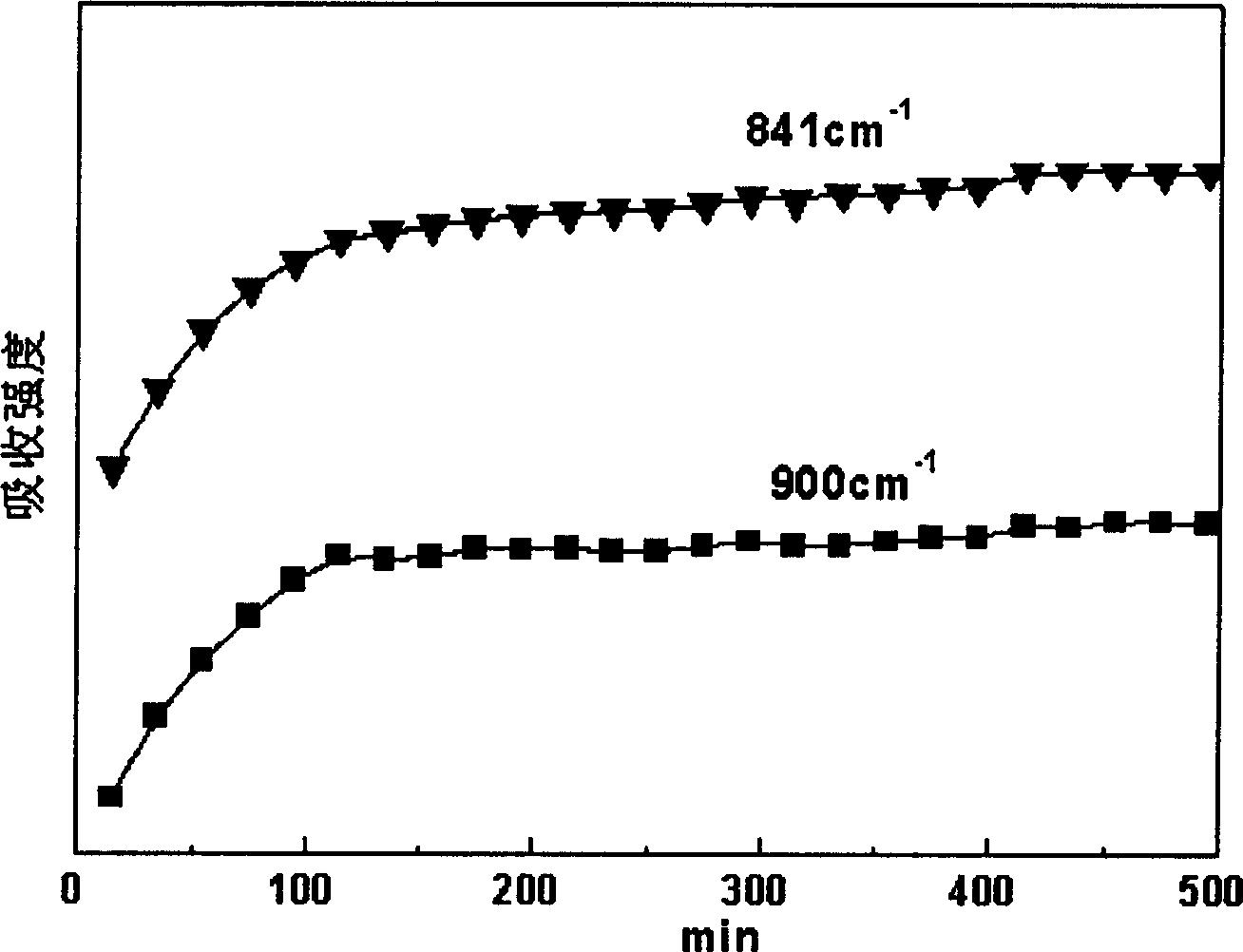
[0039] An isotactic polypropylene membrane with a diameter of 0.5 cm and a thickness of 0.1 mm was placed in an in-situ Fourier transform infrared spectrometer equipped with a high-pressure infrared cell, and then carbon dioxide was charged into the system through a high-pressure syringe pump to maintain its temperature at 100 °C. The pressure is 10MPa, making it reach the supercritical state, and keep it for 48 hours. Use infrared spectroscopy to scan the infrared spectrum every 20min. Through the analysis of the infrared spectrum, it is found that the intensity of the absorption peak corresponding to the crystallization gradually increases with time, and reaches equilibrium in about 3 hours, that is to say, supercritical carbon dioxide has an induced crystallization effect on isotactic polypropylene.
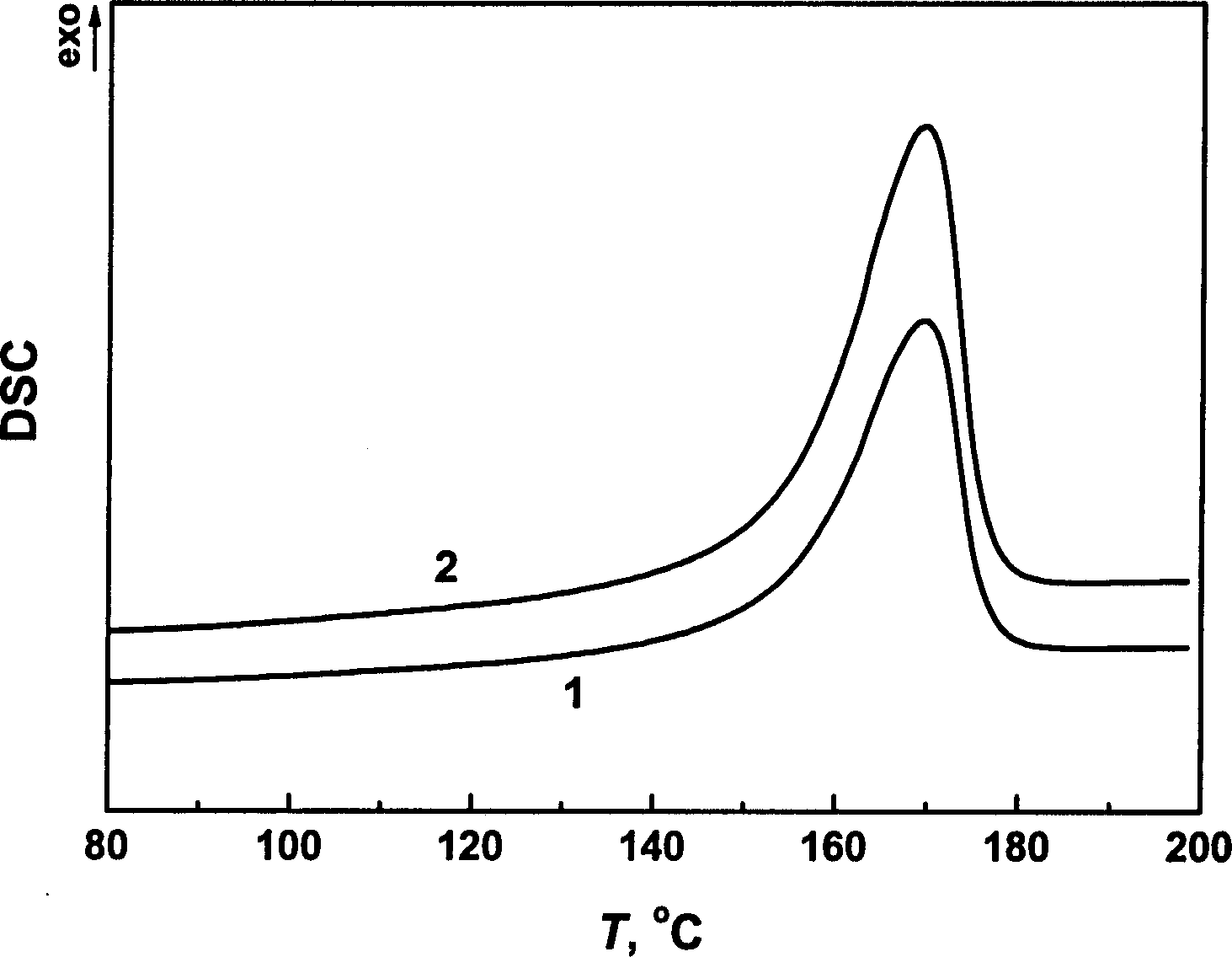
[0040] Differential scanning calorimetry (DSC) analysis of isotactic polypropylene membranes before and after the experiment showed a significant increase in crystallinity, co...
Embodiment 2
[0042]A polyethylene diaphragm with a diameter of 0.5 cm and a thickness of 0.5 mm was placed in an in-situ Fourier transform infrared spectrometer equipped with a high-pressure infrared cell, and then propane was charged into the system through a high-pressure syringe pump to maintain its temperature at 150 °C and pressure It is 15MPa, making it reach the supercritical state and keep it for 48 hours. Use infrared spectroscopy to scan the infrared spectrum every 10min. By analyzing the infrared spectrum, it is found that the intensity of the absorption peak corresponding to the crystal does not change significantly with time, that is to say, the interaction between supercritical propane and polyethylene is very weak. This phenomenon was confirmed by differential scanning calorimetry (DSC) analysis of polyethylene membranes before and after the experiment. See Figure 4 and Figure 5 .
Embodiment 3
[0044] A syndiotactic polypropylene diaphragm with a diameter of 0.5 cm and a thickness of 0.2 mm was placed in an in-situ Fourier transform infrared spectrometer equipped with a high-pressure infrared cell, and then ethylene was charged into the system through a high-pressure syringe pump to maintain its temperature at 60 °C. The pressure is 20MPa, making it reach the supercritical state, and keep it for 48 hours. Use infrared spectroscopy to scan the infrared spectrum every 5min. Through the analysis of the infrared spectrum, it is found that the intensity of the absorption peak corresponding to the unstable crystal form gradually weakens with time, and the intensity of the absorption peak corresponding to the stable crystal form gradually increases with time, and reaches equilibrium in about 2 hours, that is, Supercritical ethylene promotes the transformation of the crystal form of syndiotactic polypropylene from an unstable state to a stable state. Differential scanning c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com