Process for preparation of benazepril
A kind of amino tert-butyl ester, high-purity technology, applied in the direction of organic chemistry, etc., can solve the problems of high raw material quantity, uneconomical, etc., and achieve the effect of improving yield and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
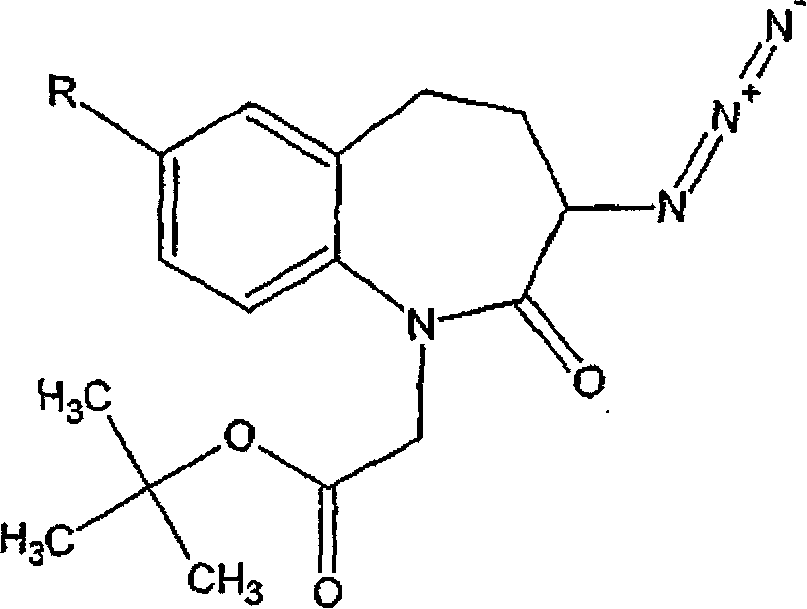
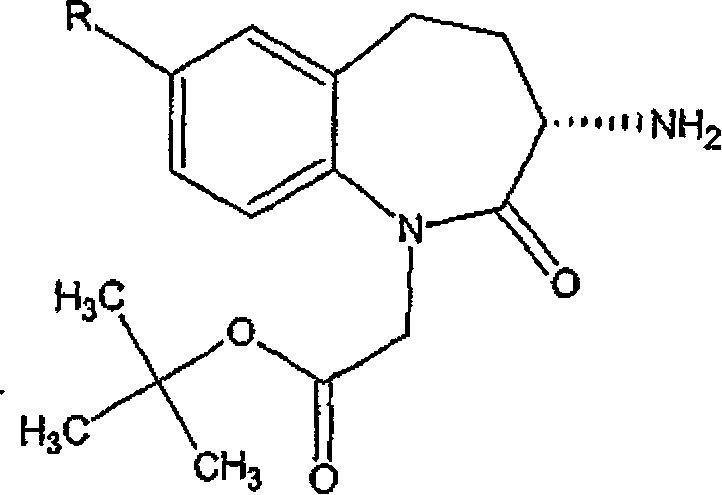
[0092] Embodiment 1: Use 10% palladium on carbon and hydrogen to prepare high-purity (±) 1-tert-butoxycarbonylmethyl-3-amino-2,3,4,5-tetrahydro-1h-[ 1) Benzazepan-2-one
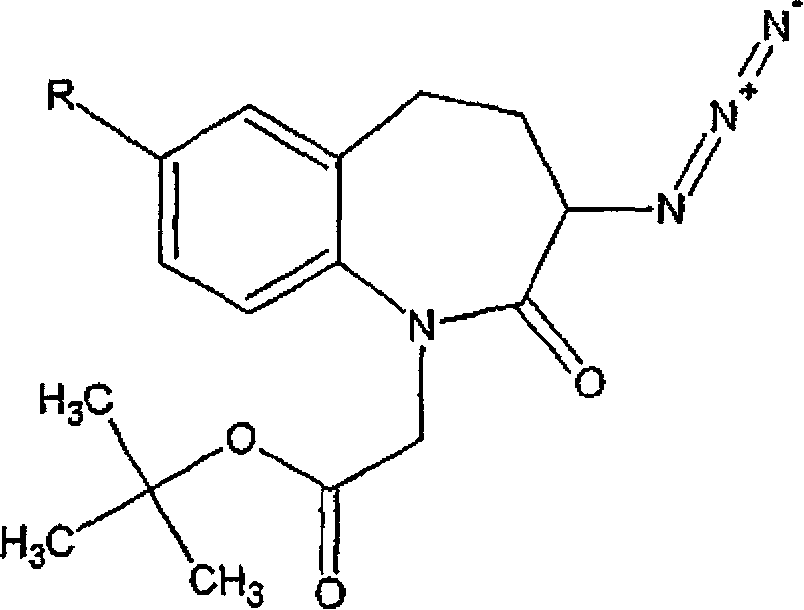
[0093]To 1-tert-butoxycarbonylmethyl-3-azido-2 shown in formula IV, 3,4,5-tetrahydro-1H-[1] benzazepan-2-ketone (5 grams , 15.8 mmol) in methanol (25 milliliters) solution, add 10% palladium on carbon (0.5 gram, 50% by weight), the compound shown in the formula IV comprises 1-tert-butoxycarbonylmethyl- 7-Bromo-3-azido-2,3,4,5-tetrahydro-1H-[1]benzazepan-2-one as impurity (7.67%). The mixture was stirred under 40-50 psi hydrogen atmosphere with periodic evacuation at room temperature. After 16 hours, the reaction mass was filtered through a bed of celite to remove palladium on carbon and the filtrate was concentrated to dryness in vacuo to afford the title product as a viscous oil which solidified on storage.
[0094] Yield: 4.5 g, 98%
[0095] Purity: 87.47%
[0096] Impurity IIa: Not detected by HPLC. ...
Embodiment 2
[0097] Example 2: Use 10% palladium on carbon and ammonium formate as the hydrogen source to prepare high-purity (±) 1-tert-butoxycarbonylmethyl-3-amino-2,3,4,5-tetrahydro shown in formula II -1h-[1]benzazepan-2-one
[0098] 1-tert-butoxycarbonylmethyl-3-azido-2,3,4,5-tetrahydro-1H-[1]benzazepan-2-one (5 grams, 15.8 mmol) in methanol (25 milliliters) solution, add ammonium formate (10.0 g, 15.75 mmol), the compound shown in the formula IV comprises 1-tert-butoxycarbonylmethyl-7-bromo- 3-Azido-2,3,4,5-tetrahydro-1H-[1]benzazepan-2-one is as impurity, and described solution comprises palladium on carbon catalyst (0.5 gram, 10%, 50 %weight). The temperature of the reaction mass was slowly raised to 40-50°C and stirred at this temperature for 16 hours. After the completion of the reaction was confirmed by TLC, the catalyst was removed by filtration, the filtrate was concentrated under vacuum, and the residue was dissolved in dichloromethane (50 mL) and water (50 mL). Separate ...
Embodiment 3
[0102] Example 3: Preparation of high-purity (±) 1-tert-butoxycarbonylmethyl-3-amino-2,3,4,5-tetrahydro-1h-[1]-benzazepine shown in formula II Heptan-2-one
[0103] Part a: Preparation of (±)1-tert-butoxycarbonylmethyl-3-azido-2,3,4,5-tetrahydro-1h-[1]benzazepane-2 represented by formula II -ketone
[0104] 1-tert-butoxycarbonylmethyl-3-azido-2,3,4,5-tetrahydro-1H-[1]benzazepan-2-one (5 grams, 15.8 mmol) in methanol (25 milliliters) solution, add Raney nickel (0.82 gram), the compound shown in the formula IV comprises 1-tert-butoxycarbonylmethyl-7-bromo-3-azide shown in the formula IVa -2,3,4,5-Tetrahydro-1H-[1]benzazepan-2-one as an impurity (7.67%). The mixture was stirred under a hydrogen atmosphere at a pressure of 40-50 psi, periodic venting, and 50-55°C. After 16 hours, the reaction mass was filtered through a bed of celite to remove Raney nickel, and the filtrate was concentrated to dryness under vacuum to afford the title product as a viscous oil which solidified o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com