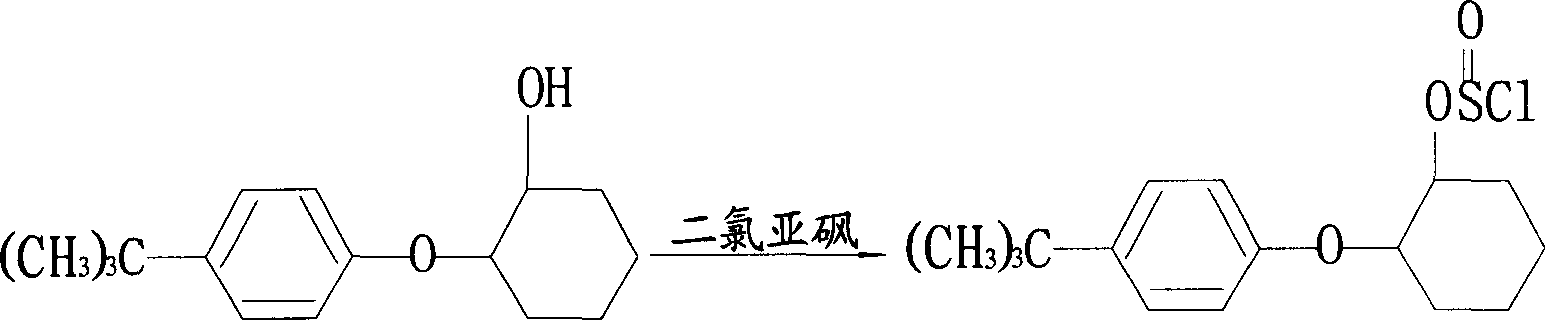
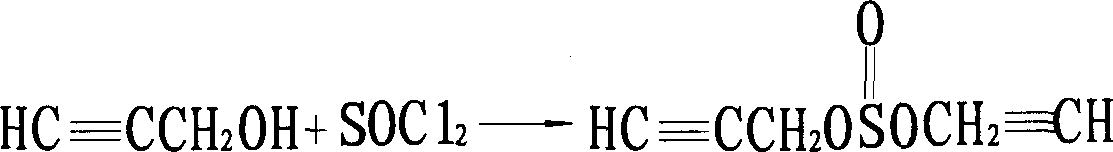Manufacturing method of high content propargite
A technology with special original medicine and high content, which is applied in acaricides, botanical equipment and methods, animal repellants, etc., can solve the problem of difficult removal of thionyl chloride, and achieve high content and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of p-tert-butylphenoxycyclohexanol: in a 100L reactor, add 15.3Kg p-tert-butylphenol (98%, 100mol), 10.9Kg epoxycyclohexane (99%, 110mol) , toluene 32.24L (99%, 300mol), reacted at 120°C for 12 hours, washed three times, each time with 20Kg of hot water at a temperature of 70°C to 80°C; at a pressure of 15mmHg and a temperature of 115°C, vacuum distillation Excess epoxycyclohexane and toluene were removed in 2.5 hours to obtain 22.89 Kg of white p-tert-butylphenoxycyclohexanol, that is, the content of the first intermediate was 98.5%, and the yield was 99.0%.
[0040] (2) Preparation of 2-(4-tert-butyl) cyclohexyl chlorosulfite toluene solution: in a 50L reactor, 11.76Kg p-tert-butylphenoxycyclohexanol (98.5%, 50mol) was dissolved in 15L In toluene, when the temperature was lowered to 40° C., 5.95 Kg of thionyl chloride (99%, 50 mol) was added dropwise, and the mixture was kept at 35° C. for 15 hours. After the heat preservation is completed, add 1.81L ...
Embodiment 2
[0044](1) Preparation of p-tert-butylphenoxycyclohexanol: in a 100L reactor, add 15.3Kg p-tert-butylphenol (98%, 100mol), 10.9Kg epoxycyclohexane (99%, 110mol) , toluene 32.24L (99%, 300mol), reacted at 120°C for 12 hours, washed three times, each time with a temperature of 80°C hot water 20Kg; at a pressure of 25mmHg, a temperature of 120°C, decompression distillation for 3 hours Excessive epoxy cyclohexane and toluene were removed to obtain 22.89 Kg of white p-tert-butylphenoxycyclohexanol, that is, the content of the first intermediate was 98.5%, and the yield was 98.5%.
[0045] (2) Preparation of 2-(4-tert-butyl) cyclohexyl chlorosulfite toluene solution: in a 50L reactor, 11.76Kg p-tert-butylphenoxycyclohexanol (98.5%, 50mol) was dissolved in 15L In toluene, when the temperature was lowered to 40°C, 7.14Kg of thionyl chloride (99%, 60mol) was added dropwise, and the mixture was kept at 38°C for 13 hours. After the heat preservation is completed, 4.36L of dichloromethane...
Embodiment 3
[0049] Repeat the same steps as described in Example 1, but from step (2): the preparation of 2-(4-tert-butyl)cyclohexyl chlorosulfite toluene solution: in a 50L reactor, 11.76Kg Tert-butylphenoxycyclohexanol (98.5%, 50mol) was dissolved in 15L of toluene, and when the temperature was lowered to 40°C, 7.14Kg of thionyl chloride (99%, 60mol) was added dropwise, and kept at 38°C for 15 hours. After the heat preservation is completed, add 6.54L dichloroethane to the reaction kettle, and at 38°C, the pressure is 25mmHg, and the vacuum distillation is carried out for 4 hours to remove the excess thionyl chloride to obtain 2-(4-tert-butyl) The toluene solution of cyclohexyl chlorosulfite, that is, 48.5 mol of the second intermediate, has a yield of 97%.
[0050] The second intermediate is condensed with propynyl alcohol to obtain 93.6% of propargite content and 92.3% of propargite yield.
[0051] All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com