Method for structuring hybridoma cell system for anti-human monoclonal antibody of hepatitis B virus and its application
A human monoclonal antibody and hybridoma cell line technology, applied in the field of bioengineering, can solve the problems of low antibody secretion and reduced antiviral effect, and achieve a large amount of secreted antibody, improve the effect of killing viruses, and have strong vitality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
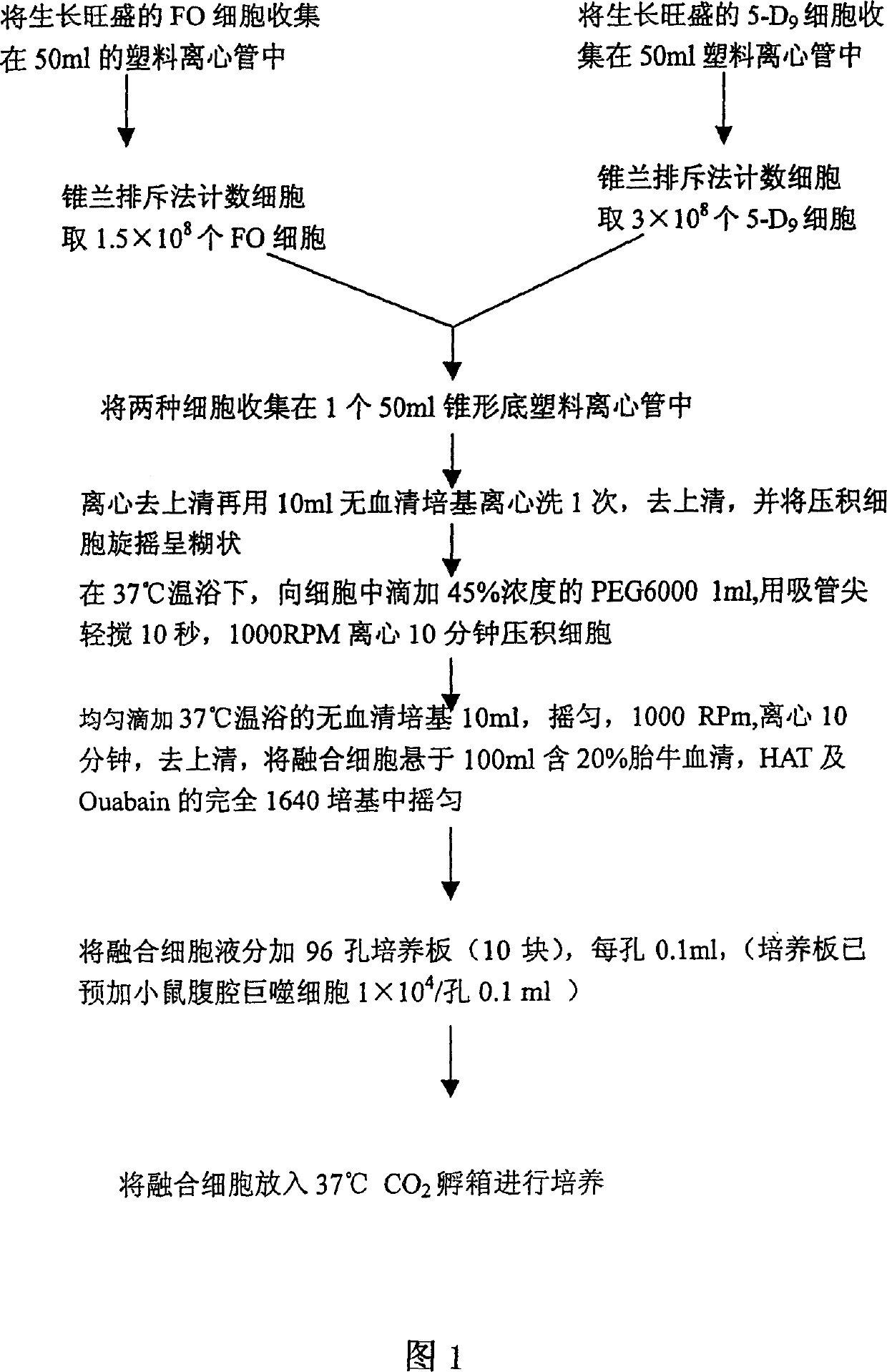
[0060] This example is a method for constructing a hybridoma cell line of anti-hepatitis B virus human monoclonal antibody (anti-HBs hMcAb).
[0061] 1. EBV transformation method to establish a transformed B lymphocyte line secreting anti-HBs hMcAb:
[0062] 1. Preparation of EBV:
[0063] ① B95-8 cells (lymphoma cell line, which can produce Epstein-Barr virus) were mixed with 2×10 5 The concentration of / ml is suspended in the RPMI1640 (complete culture medium) containing 20% fetal calf serum;
[0064] ②Cultivate the cell solution in a 37°C incubator for 7 days;
[0065] ③ Centrifuge the cultured B95-8 cell solution at 3000rpm for 10 minutes;
[0066] ④Take 5ml of B95-8 supernatant for later use.
[0067] 2. Preparation of PBL (peripheral blood lymphocytes) of anti-HBs positive young women:
[0068] ① Take 5ml of peripheral blood from anti-HBs positive young women;
[0069] ② Centrifuge the 5ml peripheral blood density gradient to separate PBL;
[0070] ③ Collect PBL, ...
Embodiment 2
[0101] This embodiment is the hybridoma cell line (6-A) of anti-hepatitis B virus human monoclonal antibody 8 Systematic assay of cell lines).
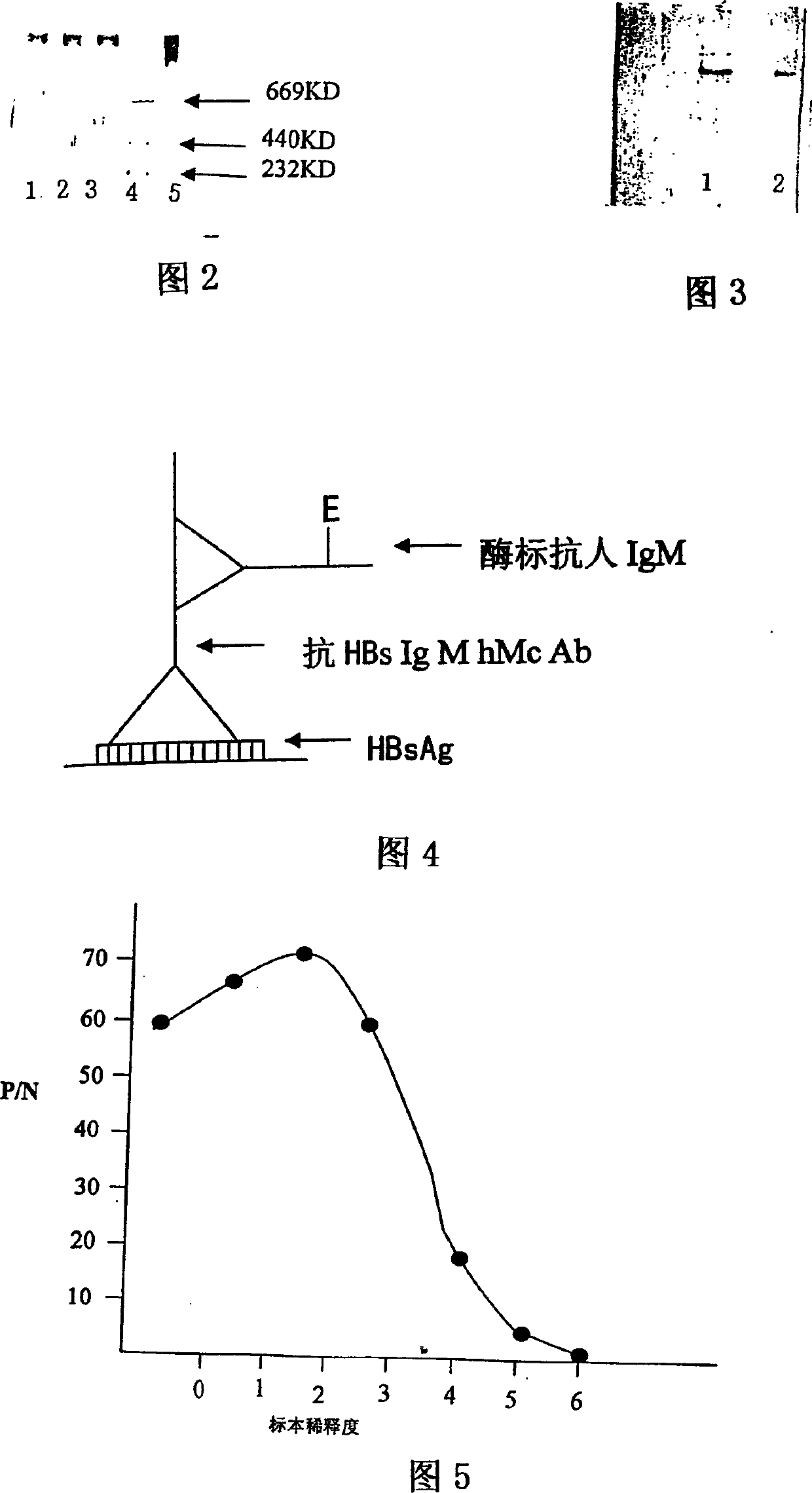
[0102] 1. Proved by ELISA indirect method, polyacrylamide gel electrophoresis and enzyme-labeled anti-human IgM immunoblotting, 6-A 8 The cells secrete anti-HBs pentameric IgM hMcAb with a molecular weight of 96×10 4 (Standard human IgM is a pentamer with a molecular weight of 96×10 4 ), has strong specificity to HBsAg (hepatitis B surface antigen), and has no cross-reaction with other hepatitis B markers and some other viruses. See Figures 2, 3, 4, 5 and Tables 1 and 2.
[0103] Dilution
1
3
9
27
81
243
729
2187
6561
OD 3
3.37
3.20
2.33
0.83
0.33
0.20
0.17
0.12
0.11
P / N
63.6
60.4
43.9
15.6
6.2
3.7
2.8
2.4
2.2
[0104] Negative control: OD ...
Embodiment 3
[0124] This example is the production process of anti-HBs pentameric IgM hMcAb.
[0125] 1. Animal method produces anti-HBs pentamer IgM hMcAb, the procedure is as follows:
[0126] Cultivate enough 6-A8 cells in a rotating glass culture flask, inject 0.5ml of pristane into each nude mouse intraperitoneally, and inject 1×107 6-A8 cells into the nude mice after 1 week of pristine injection, and feed them continuously The nude mice were injected with cells for 2 weeks. After the abdominal cavity of the nude mice was extremely distended, the nude mice were sacrificed, and the 6-A8 cell solid tumors were removed by laparotomy, and stored in a -40°C low-temperature refrigerator for later use;
[0127] Then carry out the separation and purification of anti-HBs pentamer IgM hMcAb: take 1 gram of solid tumor, cut it into pieces, grind it, add 3ml of 0.01M PH7.4 PBS, ultrasonic homogenate for 15 minutes, centrifuge at 10000rpm for 25 minutes at low temperature, collect the supernatant,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com