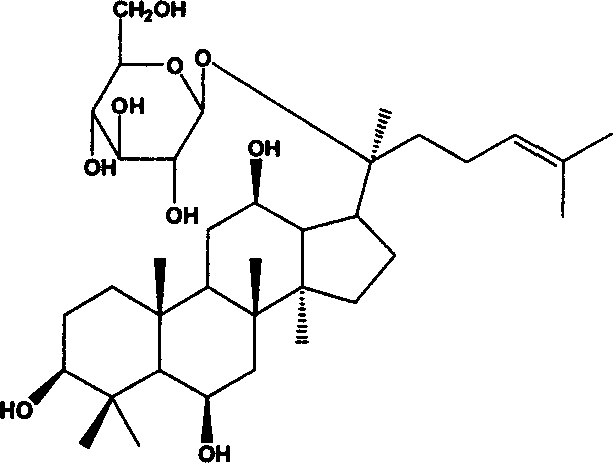
Ginsenoside F1 medicinal uses
A technology of ginsenosides and drugs, applied in the field of medicine, can solve the problems of high fatality rate, low cure rate, difficult treatment, etc., and achieve the effect of small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0047] Preparation Example 1 Ginsenoside F 1 preparation of
[0048] 500mg ginsenoside Re and 150mg naringinase were dissolved in 50ml of phosphoric acid-citric acid buffer (pH 4.5, ionic strength 0.01M, containing 10% ethanol), hydrolyzed in a water bath at 40°C for 7 days; the reaction solution was centrifuged Collect the precipitate; wash the precipitate with 10ml of water, wash 3 times (10ml×3), combine the washing liquid with the mother liquor, concentrate to 50ml for reuse; wash the precipitate with 10ml ethanol, wash 5 times (10ml×5), combine the ethanol solution, Evaporate to dryness and collect the residue to obtain ginsenoside F 1 301 mg. Determination of Ginsenoside F by HPLC 1 The content is 63%.
preparation example 2
[0049] Preparation Example 2 Ginsenoside F 1 preparation of
[0050] 500mg Ginsenoside Rg 1 , 150mg of naringinase was dissolved in 50ml of glycine-HCl buffer (pH 4.5, ionic strength 0.01M, containing 10% dimethyl sulfoxide), and hydrolyzed in a water bath at 40°C for 7 days; the reaction solution was centrifuged to collect the precipitate ; Wash the precipitate with 10ml of water, wash 3 times (10ml × 3), combine the lotion with the mother liquor, concentrate to 50ml for reuse; wash the precipitate with 10ml of ethanol, wash 5 times (10ml × 5), combine the ethanol solution, evaporate to dryness , collect the residue to get ginsenoside F 1 325mg. Determination of Ginsenoside F by HPLC 1 The content is 84%.
preparation example 3
[0051] Preparation Example 3 Ginsenoside F 1 preparation of
[0052] 500mg Ginsenosides Re and Rg 1 The mixture, 150mg of naringinase was dissolved in 50ml of acetic acid-sodium acetate buffer solution (pH 4.5, ionic strength of 0.01M, containing 10% formamide), and hydrolyzed in a water bath at 40°C for 7 days; the reaction solution was collected by centrifugation Precipitate; wash the precipitate with 10ml of water, wash 3 times (10ml×3), combine the lotion with the mother liquor, concentrate to 50ml for reuse; wash the precipitate with 10ml ethanol, wash 5 times (10ml×5), combine the ethanol solution, distill Dry and collect the residue to get ginsenoside F 1 310mg. Determination of Ginsenoside F by HPLC 1 The content is 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com