Cefditoren pivoxil dry suspensoid and its preparing process
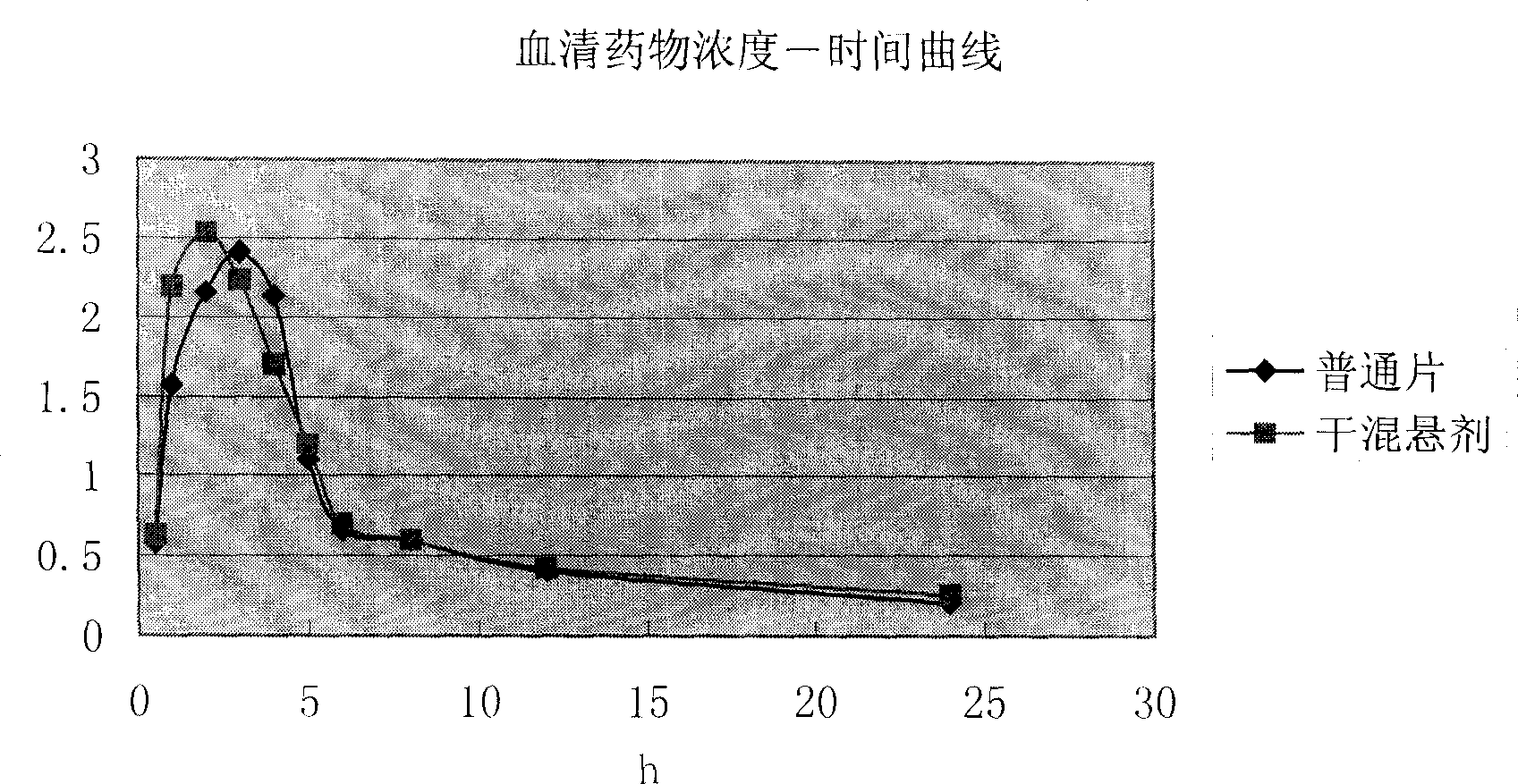
A technology of cefditoren pivoxil and dry suspension, which is applied in the directions of pharmaceutical formulas, medical preparations without active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of cefditoren pivoxil dry suspension. Research and reports, single dosage form, unable to meet the needs of medication, etc., to achieve the effect of improving medication compliance, good taste, and facilitating drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
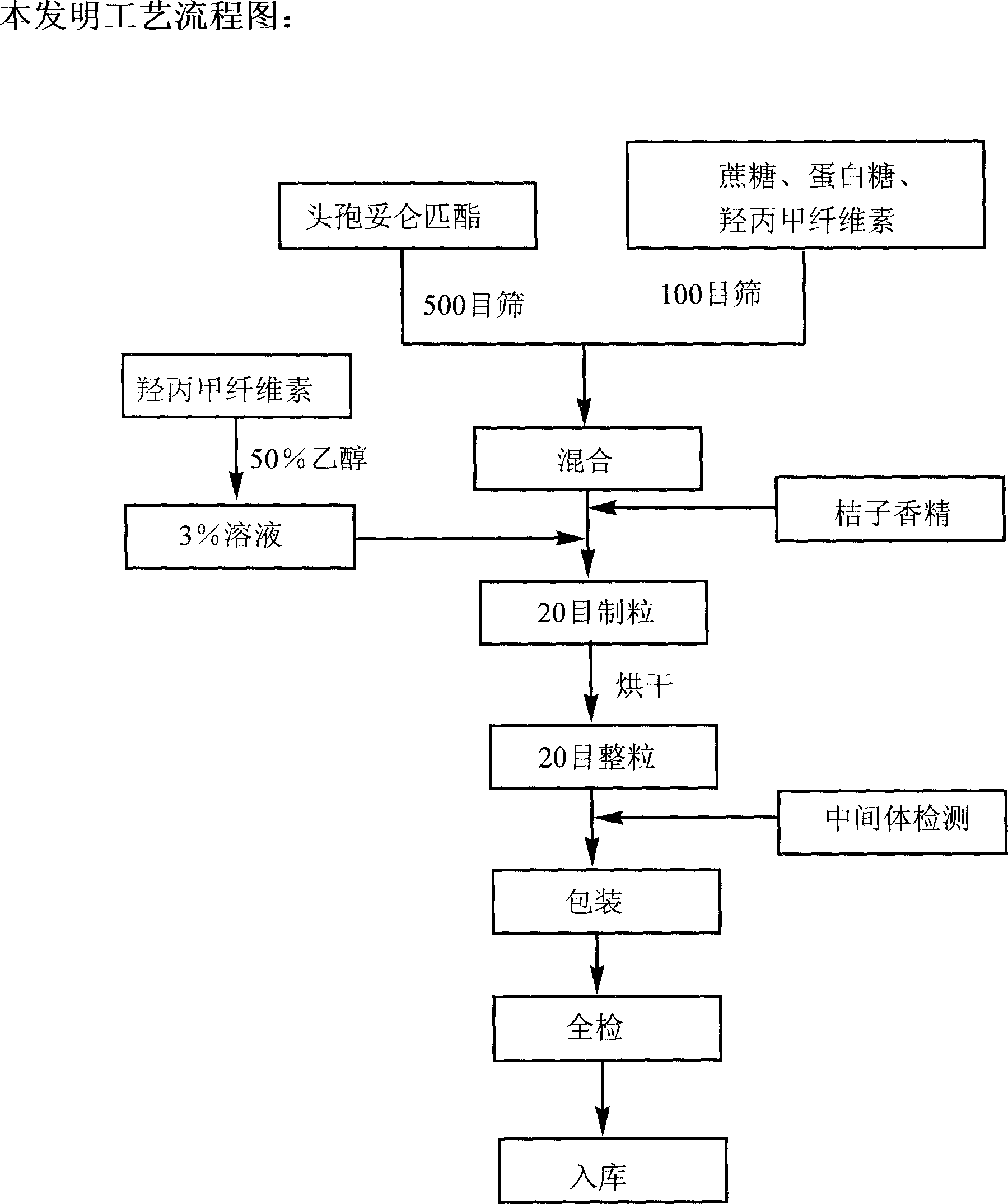
[0039] Calculated by preparing 1000 bags, the composition and proportioning of raw and auxiliary materials are:
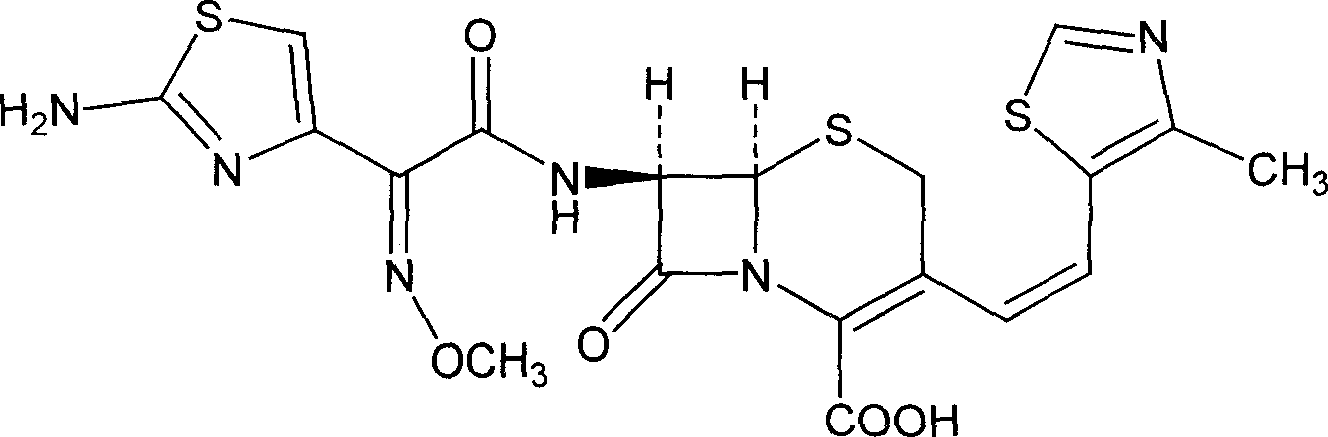
[0040] Cefditoren pivoxil 100g
[0041] Sucrose 1900g
[0042] Hypromellose 60g
[0044] Orange flavor 30g
[0045] Make 1000 bags
[0046] Preparation:
[0047] ①Cefditoren pivoxil passed through a 500-mesh sieve, and the rest of the excipients passed through a 100-mesh sieve.
[0048] ② Weigh 1 / 6 prescription amount of hypromellose and 50% ethanol solution to make a 3% solution for later use.
[0049] ③ Weigh the prescribed amount of cefditoren pivoxil, sucrose, protein sugar and remaining hypromellose, mix evenly, add the above-mentioned adhesive and soft material made of orange essence, granulate with 20 mesh, blow dry at 60°C, and dry for 20 Whole grain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com