Medicine containing bacillus tubercle vaccine
A technology of Mycobacterium tuberculosis and nucleic acid vaccines, which is applied in the direction of antibacterial drugs, medical formulas, bacterial antigen components, etc., can solve the problems of easy loss of protection and poor use effect of adults, and achieve improvement of lung pathological conditions, easy production, The effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, the preparation of tubercle bacillus vaccine package medicine
[0030] 1. Construction of eukaryotic expression vector
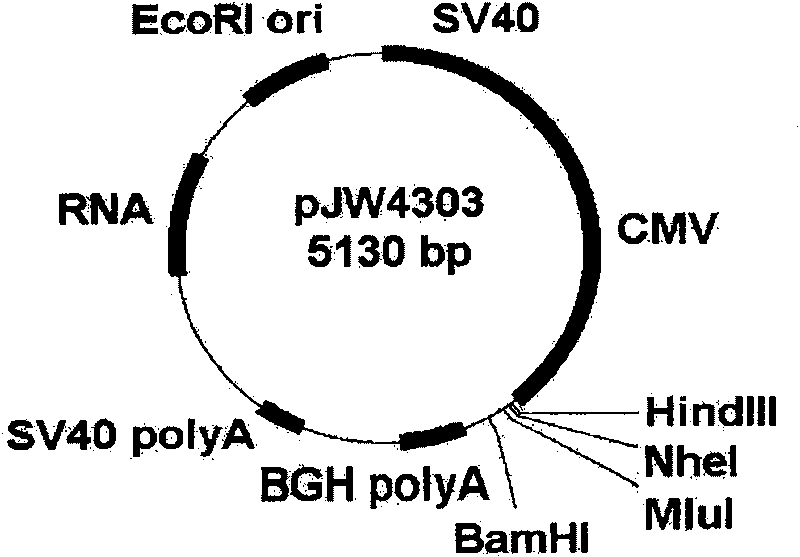
[0031] The Ag85B, MPT-83 and MPT64 genes of M. tuberculosis were amplified by PCR and cloned into the eukaryotic expression vector pJW4303 for expression in eukaryotic cells. Gene Bank accession numbers are: Ag85B: X62398; MPT-83: X94579; MPT64: U34849. The Mycobacterium tuberculosis genomic DNA extracted by conventional methods was used as a template, and Ag85B, MPT-83 and MPT64 genes were amplified by PCR and directionally cloned into the pJW4303 vector (the physical map is as follows: figure 1 Shown) downstream of the tissue plasminogen activator (TPA) signal sequence, forming a fusion protein. The primer sequences used for amplification are as follows:
[0032] Ag85B: 5'-AAATGGGGCACAGCTAGCCATATGACAGACGTGAGCC-3' and
[0033] 5'-ACTAGGATCCTAAGCAACCTTCGGTTGATCCCGTCAGC-3';
[0034] MPT64: 5'-TAGAGTACTGCTAGCGTGCGCATCAAGATCTTC-3' and ...
Embodiment 2
[0041] Embodiment 2, the effect experiment of tubercle bacillus vaccine package medicine
[0042] Divide 40 cows into 4 groups, and carry out the following treatment respectively: the first group is the DNA vaccine group, each 500 μ g of the plasmids pJAg85B, pJMPT-83 and pJMPT64 prepared in Example 1 are dissolved in 1500 ml of normal saline or PBS, and mixed , divided into three parts on average, three times for intramuscular immunization, the immunization dose was 1500 μl / head / time, and the time points were the 0th week, the 4th week, and the 8th week; In the 0th week, the 4th week, and the 8th week, the vaccine I, vaccine II and vaccine III of the Mycobacterium tuberculosis vaccine kit were injected three times respectively; the immunization doses of vaccine I, vaccine II and vaccine III were all 1500 μl / head / time, 1500 μl / head / time and 500μl / head / time; the third group was the BCG group, which received subcutaneous injection of 1×10 6 CFU of BCG (purchased from China In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com