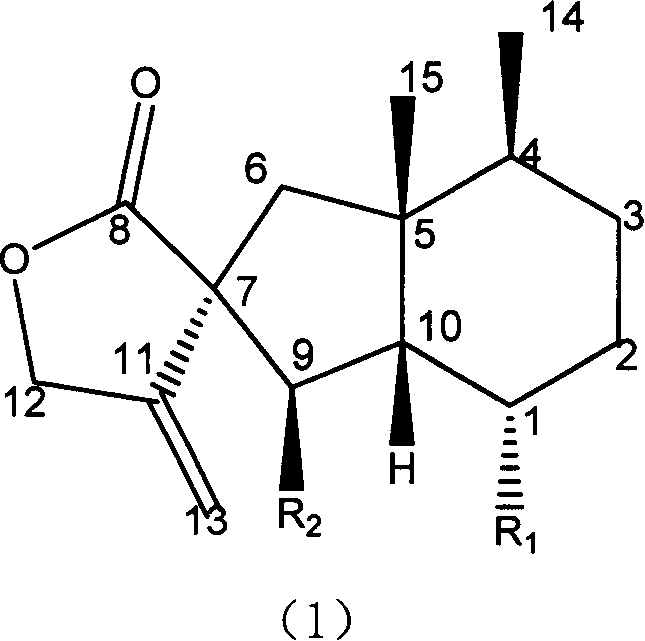
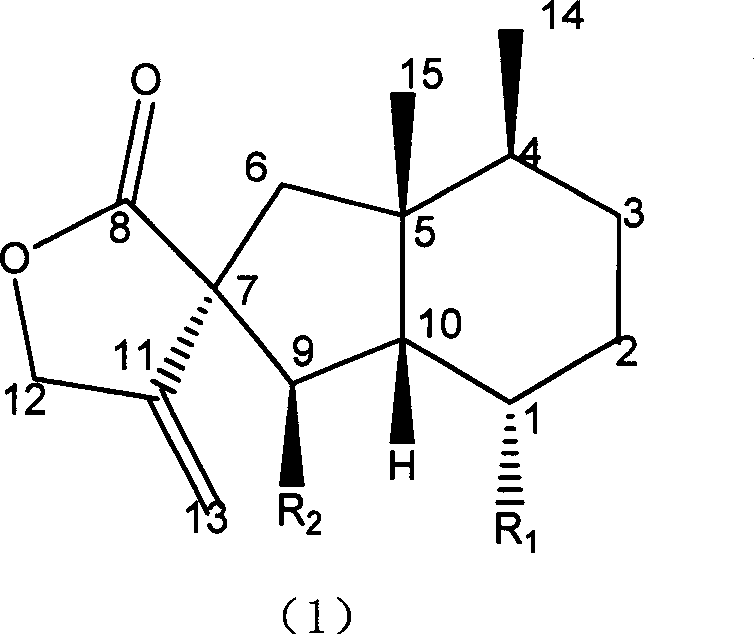
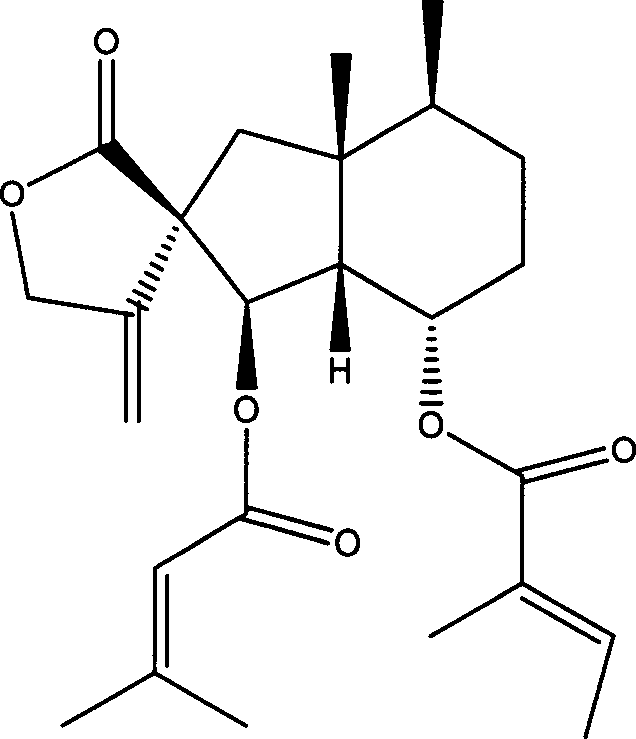
Fukinanolide compounds and pharmaceutical application thereof
A technology of butterburide and compound, applied in the field of chemical structure of four new butterburide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071]35Kg of dry leaves of Butterbur trichomes were extracted three times by reflux with ethanol, the amount of ethanol was 5 times, 2 times and 1 time of the rhizome respectively, and the extraction time was 2 hours, 1 hour and half an hour respectively. The extract was concentrated under reduced pressure and dissolved in water to make a suspension, which was sequentially extracted with petroleum ether and ethyl acetate to obtain petroleum ether extract and ethyl acetate extract respectively. The ethyl acetate extract was subjected to silica gel column chromatography (chromatographic silica gel 200-300 mesh, Shandong Yantai Zhifu Huangwu Silica Gel Development and Experimental Factory), washed with n-hexane-ethyl acetate (8:1-1:1) solvent system According to thin layer chromatography monitoring, the eluate was combined into thirteen parts. The fourth part was subjected to silica gel column chromatography, eluting with n-hexane-ethyl acetate (20:1), and the eluate was concent...
Embodiment 2
[0074] Add 10 liters of ethanol to 5 kg of dried rhizomes of Butterbur trichomes, and percolate 3 times. The percolates were combined and concentrated under reduced pressure to a certain volume to obtain the crude extract of total butterbur lactones. Add water to dissolve the crude butterbur lactone, and then pass it through a macroporous adsorption resin (D101 type, Tianjin Zhengtiancheng Clarification Technology Co., Ltd., or other brands, such as AB-8, D4020, 860021, HP20, SP825 etc.), rinse with water or dilute ethanol, and then rinse with concentrated ethanol, and recover the rinsed portion of concentrated ethanol under reduced pressure to obtain butterbur lactone mixture. This mixture was subjected to silica gel column chromatography, and was eluted with petroleum ether-acetone (50-5: 1) to obtain Homofukinolide (7.9 mg), butterbur lactone-Ia (29 mg) and butterbur lactone-IIa ( 65mg), Butterburide-B(1.6g), Butterburide-IIIa(2.8g), Butterburide-IVa(2.9g), Butterburide-D(...
Embodiment 3
[0076] 5Kg of dried rhizomes of Butterbur were extracted three times under reflux with methanol, the dosage of methanol was 5 times, 2 times and 1 times of the rhizomes respectively, and the extraction time was 2 hours, 1 hour and half an hour respectively. The extract was concentrated under reduced pressure and dissolved in water to make a suspension, which was sequentially extracted with petroleum ether and ethyl acetate to obtain petroleum ether extract and ethyl acetate extract respectively. The petroleum ether extract was eluted with a gradient of n-hexane-ethyl acetate (20:1-1:1) by silica gel column chromatography (chromatographic silica gel 200-300 mesh, Shandong Yantai Zhifu Huangwu Silica Gel Development and Experimental Factory), Compound butterbur lactone A (6.1mg), Homofukinolide (6.9mg), butterbur lactone-Ia (5mg) and butterbur lactone-IIa (6.3mg), butterbur lactone-Fa were obtained sequentially (6.2mg), butterbur lactone-Fb (26.9mg), butterbur lactone-I (14.3mg)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com