Extraction separation purification and identification of flavonoid monomers in oriental blueberry melanin
A technology of flavonoids and urchins, applied in the direction of organic chemistry, etc., can solve the problems of increased elution time, unsatisfactory separation effect, and poor component separation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Structural Identification of Compound A
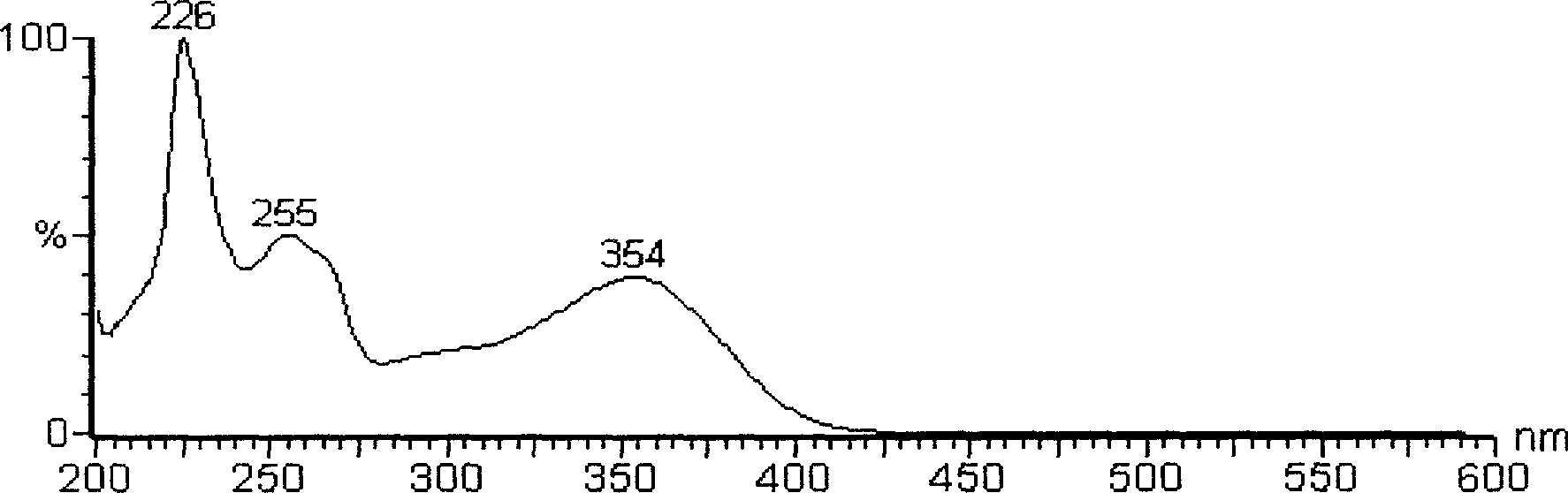
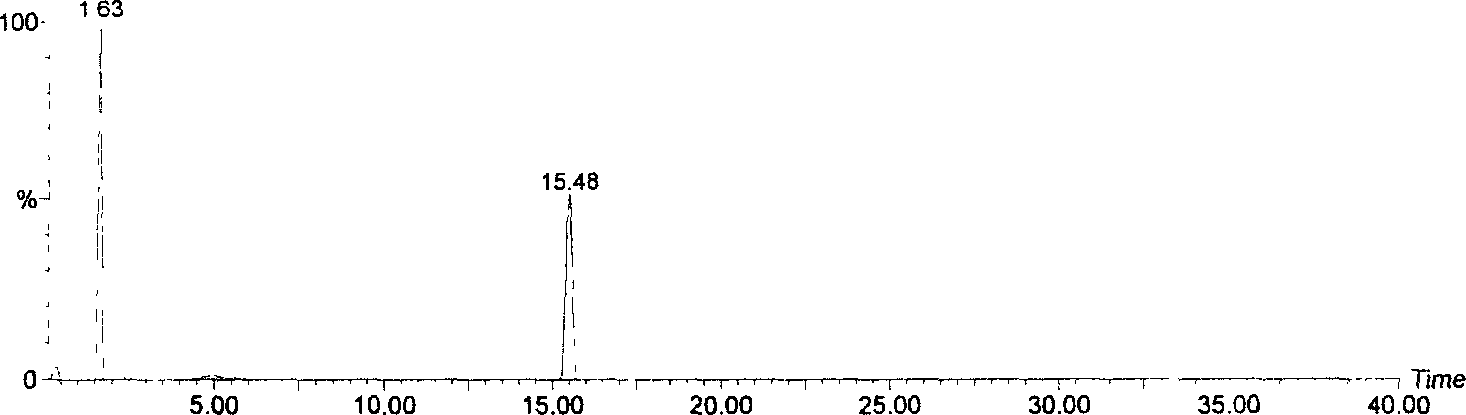
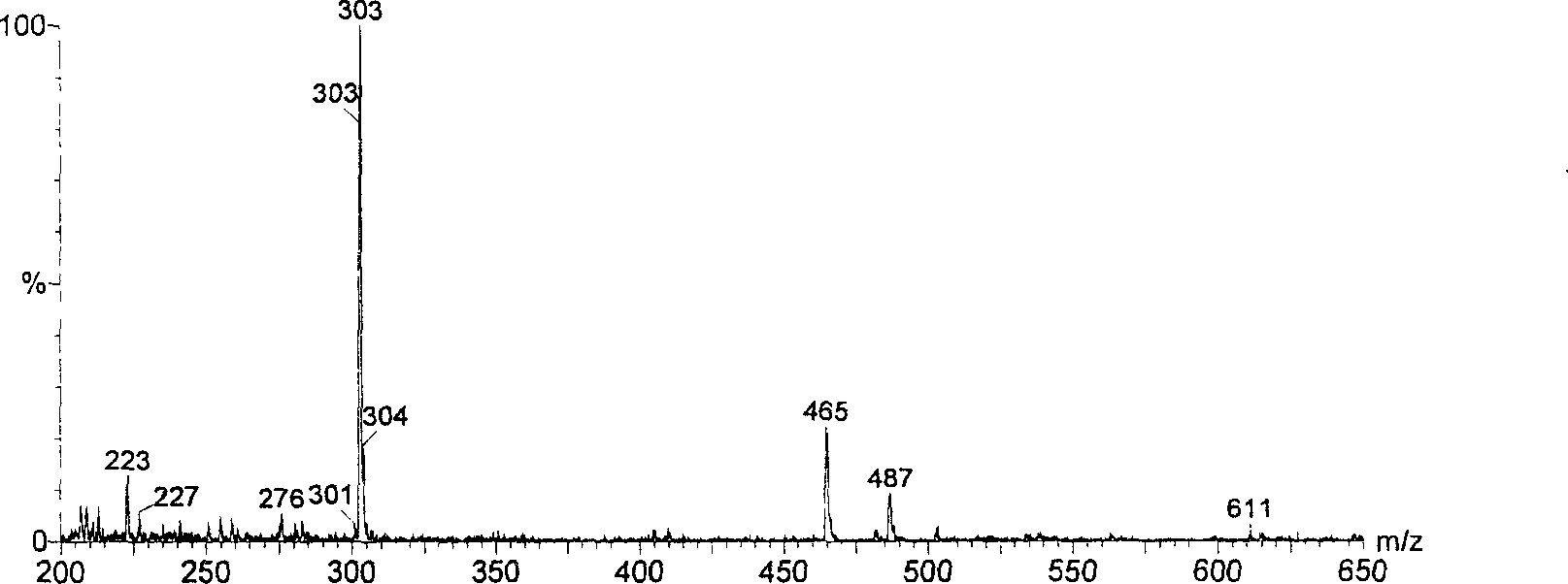
[0030] Due to the small amount of this substance, further purification is more difficult, so only a simple analysis is carried out. The substance is yellow in ammonia smoke, and it turns red in the hydrochloric acid-zinc powder test, and there is a red precipitate after standing; the lead salt precipitation test results show an obvious red precipitate; AlCl 3 Experimentally, it produces a yellow color and fluoresces under ultraviolet light. For wavelength scanning of this material, see figure 1 , it can be seen from the figure that this compound has the characteristic absorption of flavonoids. Carry out HPLC purity measurement to this material, specific result sees figure 2 , it can be seen that the purity of compound A is higher, and from image 3 It can be seen from the figure that the flavonoid aglycone molecular weight of this compound is 302, and the molecular weight is 464, which is presumed to be connected ...
Embodiment 2
[0031] Example 2 Structural Identification of Compound B
[0032] This substance is light yellow powder, easily soluble in methanol and ethanol. Ammonia fumigation is yellow, so it is a flavonoid compound. From Figure 4 and Figure 5It can be seen that the relative molecular mass of the sample should be 302, which is consistent with the relative molecular mass of quercetin, which can preliminarily indicate that the two are the same substance. Hydrogen spectrum ( 1 H-NMR): δ7.67 (1H, H-2'), δ7.53 (1H, d, d, J=8.5Hz, 1.6Hz, H-6'), δ6.88 (1H, d, J =8.5Hz, H-5'), δ6.19 (1H, S, H-6), δ6.41 (1H, S, H-8).
[0033] Through the analysis of the above spectrum, and according to the relevant references, it can be concluded that the extracted compound B is quercetin. The molecular structural formula is as follows:
[0034]
Embodiment 3
[0035] Example 3 Structural Identification of Compound C
[0036] This substance is a light yellow powder, yellow in ammonia smoke, easily soluble in methanol and ethanol. Hydrogen spectrum ( 1 H-NMR): δ8.02 (2H, d, J=7.1, H-2', 6'), δ7.6 (1H, dd, J=7.1, 6.8, H-4'), δ7.56 ( 2H, dd, J=7.1, 6.8, H-3', 5'), δ6.9 (1H, S, H-3), δ6.5 (1H, d, J=2.0, H-8), δ6 .2 (1H, d, J=2.0, H-6). From 1 It can be seen from H-NMR that there are 8 hydrogens in the single ring region, and the two hydrogens of δ6.5 and δ6.2 are coupled with each other. From the J value, it should be the meta hydrogen on the single ring, indicating that the benzene ring is 4 bits to replace. There are 5 hydrogens at δ8.02, δ7.6 and δ7.56. Judging from the peak shape and J value, it should be a spin system, so it is a monosubstituted benzene ring.
[0037] carbon spectrum ( 13 C-NMR): δ181.8(C4), δ164.4(C2), δ163.1(C8'), δ161.4(C7), δ157.4(C5), δ131.9(C1'), δ130 .7(C4'), δ129.0(C2', C6'), δ126.3(C3', C5'), δ105.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com