Novel crystalline forms of a phosphoric acid salt of a dipeptidyl peptidase-iv inhibitor
A technology of dihydrogen and anhydrous crystals, applied in the treatment of symptoms requiring DPP-IV inhibitors, crystallization of new solvates of dihydrogen phosphate, and crystal anhydrates, which can solve undisclosed problems and achieve simplified processing, The effect of improving physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072]
[0073] (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4, 3-α]Pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine dihydrogen phosphate Mixture of salt anhydrous forms I and III
[0074] Preparation of 3-(trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-α]pyrazine salt Acid (1-4)
[0075] Process 1
[0076]
[0077] Step A : Preparation of bishydrazide (1-1)
[0078] Hydrazine (20.1 g, 35 wt% in water, 0.22 mol) was mixed with 310 mL of acetonitrile. 31.5 g of ethyl trifluoroacetate (0.22 mol) was added over 60 min. The internal temperature of the solution was increased from 14°C to 25°C. The resulting solution was aged at 22-25°C for 60 min. The solution was then cooled to 7°C. 17.9 g of 50 wt% liquid NaOH (0.22 mol) and 25.3 g of chloroacetyl chloride (0.22 mol) were added simultaneously below 16°C over 130 min. After the reaction was completed, the mixture was vacuum distilled to remove water and ethanol at 27-30°C u...
Embodiment 2
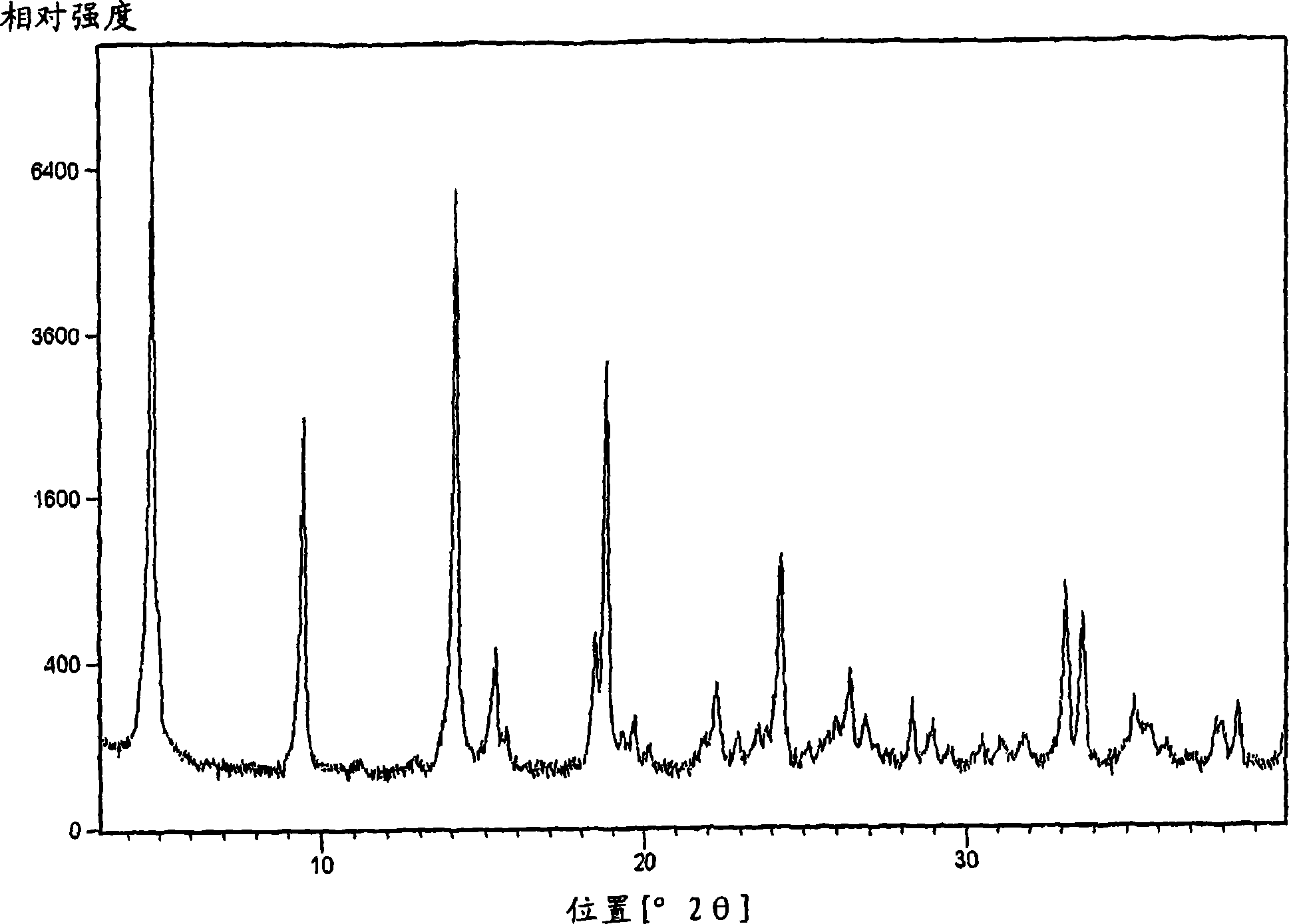
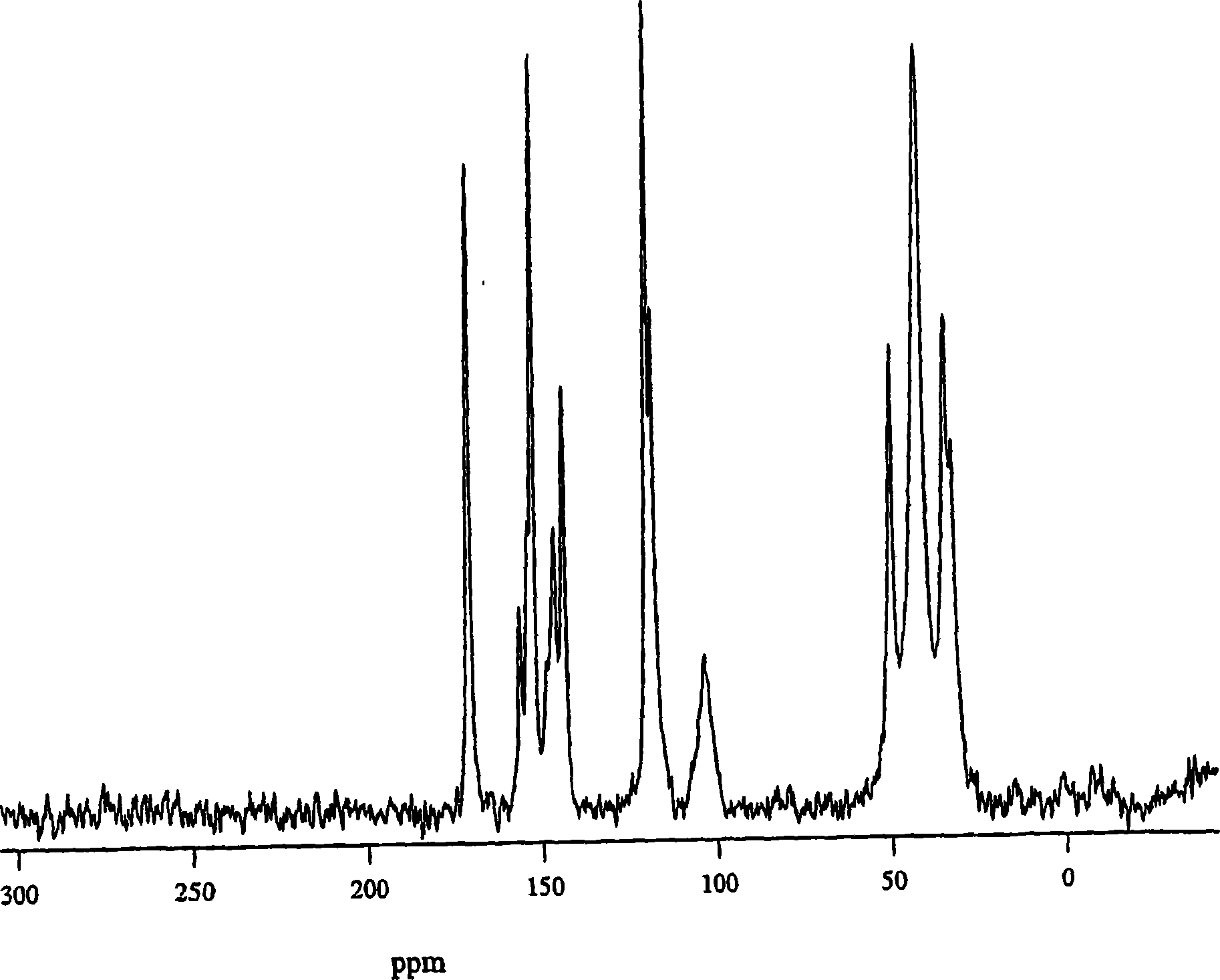
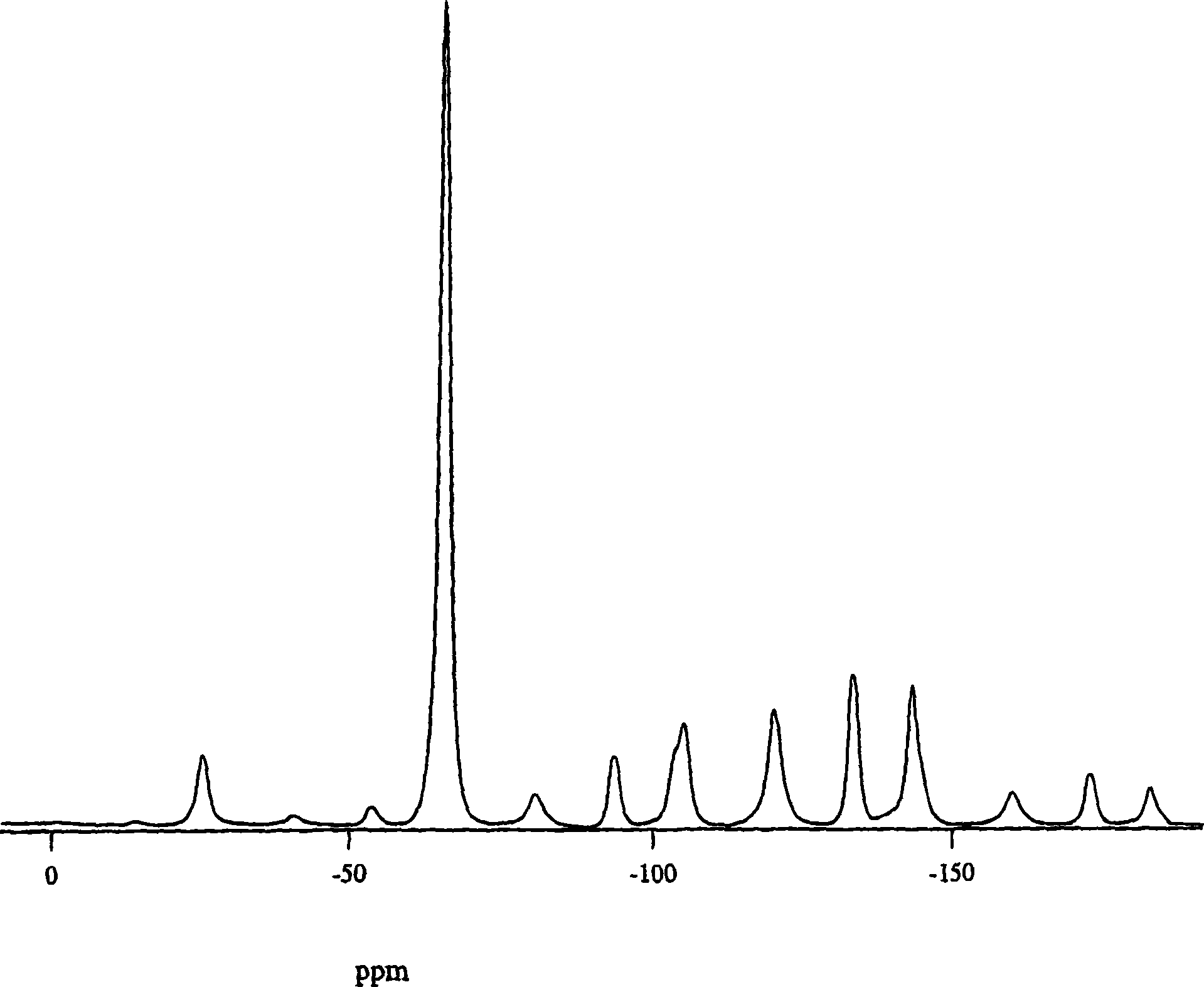
[0143] (2R) 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-α]pyrazine-7(8H )-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine free base 2-5 in isoamyl alcohol (~200 mg / g) was charged to the crystallizer. A seed crystal was then added, followed by a mixed solution of 96% isoamyl alcohol and 4% water. The mixture was aged before heating to about 50°C. A mixed solution of about 1 equivalent of phosphoric acid in 96% isoamyl alcohol and 4% water (to obtain a final batch concentration of 85 mg / g) was added to the slurry to crystallize Form I. The slurry was aged, followed by cooling to room temperature. The solid was filtered and washed with isoamyl alcohol. The wet solids were dried at 75-80°C. These crystalline solids were determined by X-ray powder diffraction and solid-state NMR spectroscopy to be a mixture of anhydrous forms I and III, with form I predominating.
[0144] X-ray powder diffraction method is widely used in characterizing molecular structure, crystalli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
| Exothermic temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com