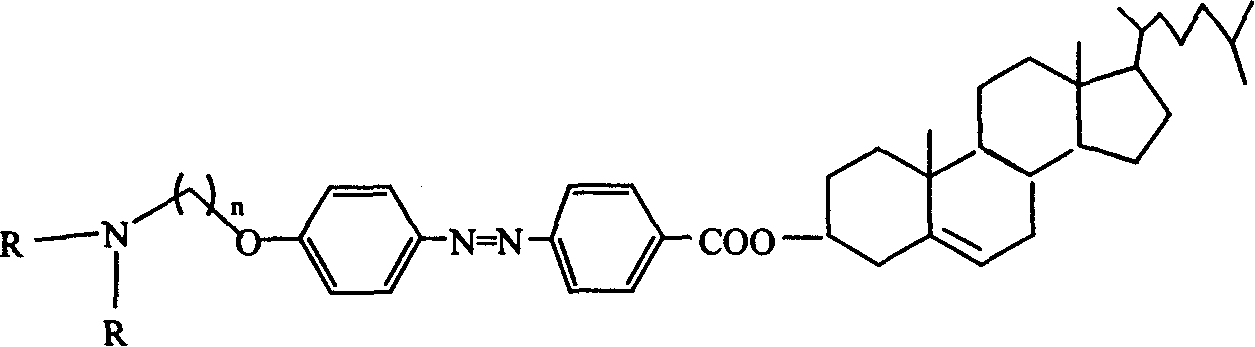
Cholesterol derivative containing azobenzene group, and its synthesizing method and use
A technology of cholesterol derivatives and azobenzene groups, applied in the field of cholesterol derivatives, can solve problems such as poor stability, and achieve the effects of easy operation and less harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
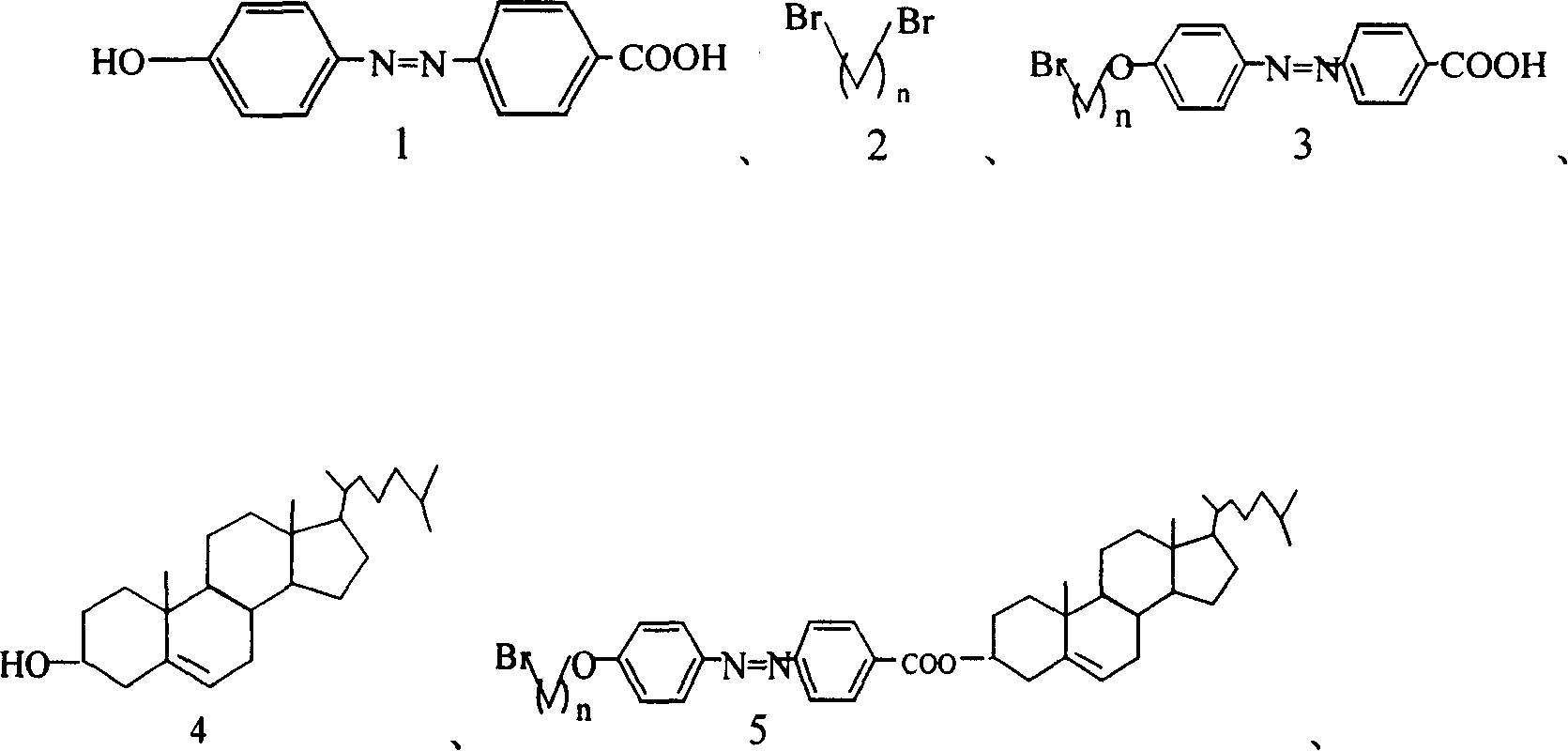
[0025] Dissolve 8mmol of compound 1 in 200ml of anhydrous acetone, add 18mmol of anhydrous K 2 CO 3 , 2mmol KI or 18-crown-6, and 32mmol compound 2 were refluxed for 36h and filtered, the filtrate was spin-dried and separated by flash column chromatography to obtain compound 3 (90%).
[0026] C 17 h 17 N 2 o 3 Br
[0027] 1 H NMR (300MHz, DMSO, ppm): 8.14-8.11 (2H, d, J=9Hz), 7.94-7.90 (4H, dd, J=6Hz), 7.17-7.14 (2H, d, J=9Hz), 4.16 -4.12(2H, t, 6Hz), 3.65-3.61(2H, t, 6Hz), 2.02-1.86(4H, m).
[0028] MS(EI): 424(M + +47, 100%), 378 (M + +1, 49.16%), 376 (M + -1, 49.71%), 183 (M + -194, 56.65%), 133 (M + -244, 28.83%), 121 (M + -244, 25.66).
[0029] IR: 1681, 1602, 1581, 1501, 1501, 1427, 1277, 1248, 1142.
Embodiment 2
[0031] Add 6mmol of compound 3 to 100ml of dichloromethane, and then dropwise add 50ml of a dichloromethane solution containing 7mmol of dicyclohexylcarbodiimide. After the solution is clarified, add dropwise 50ml of a dichloromethane solution containing 7mmol of compound 4, and react at room temperature for 12h. Filter, extract with 0.1N hydrochloric acid, then wash with saturated NaHCO 3 Extraction with aqueous solution, and finally extraction with bromine water, the organic phase was spin-dried, and separated by flash column chromatography to obtain compound 5 (55%).
[0032] C 44 h 61 N 2 o 3 Br
[0033] 1 H NMR (300MHz, CDCl 3 , ppm): 8.18-8.15 (2H, d, J = 9Hz), 7.96-7.88 (4H, dd, J = 9Hz), 7.02-6.99 (2H, d, J = 9Hz), 5.44-5.42 (1H, m ), 4.94-4.83(1H, m), 4.11-4.07(2H, t, J=6Hz), 3.53-3.49(2H, t, J=6Hz), 2.50-0.69(47H, m).
[0034] MS (MALDI): 745.4 (M + ), 747.4 (M + +2).
[0035] IR: 2939, 1722, 1708, 1602, 1500, 1468, 1282, 1257, 1143, 1116.
Embodiment 3
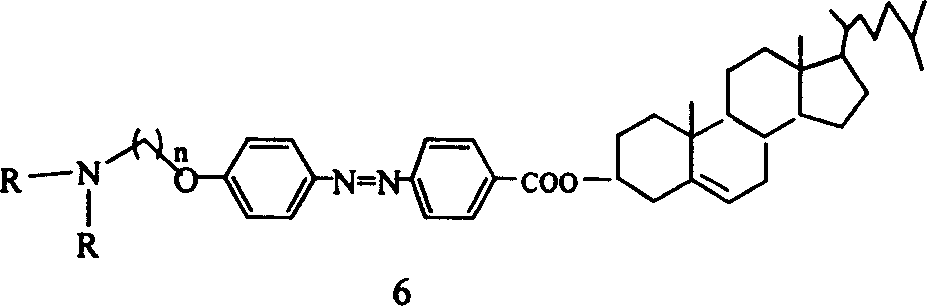
[0037] 3 mmol of compound 5a and 5 ml of diethylamine were refluxed in chloroform for 3 days, filtered after cooling, washed with chloroform, and separated by flash column chromatography to obtain compound 6a (50%). where n=4.
[0038] C 48 h 71 N 3 o 3
[0039] 1 HNMR (300MHz, CDCl 3 , ppm): 8.19-8.16 (2H, d, J = 9Hz), 7.97-7.89 (4H, dd, J = 9Hz), 7.02-6.99 (2H, d, J = 9Hz), 5.44 (1H, m), 4.93-4.83(1H, m), 4.11-4.07(2H, t, J=6Hz), 3.23-3.16(6H, m), 2.51-0.69(53H, m)
[0040] MS (MALDI): 737 (M + ), 738 (M + +1), 739(M + +2)
[0041] IR: 2926, 2854, 1713, 1601, 1583, 1502cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com