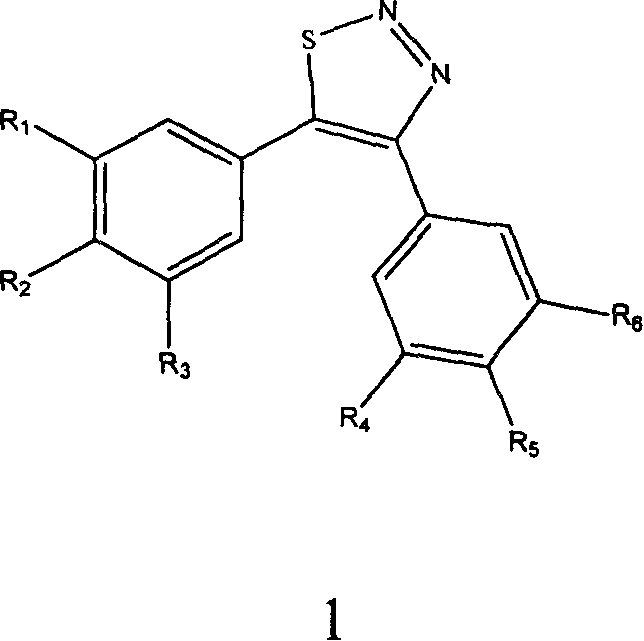
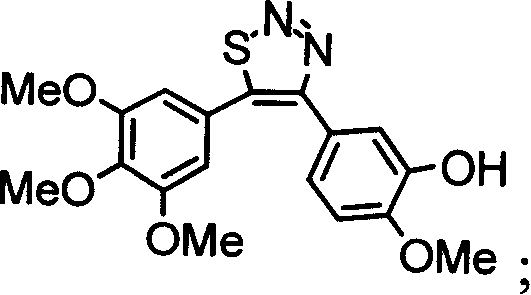
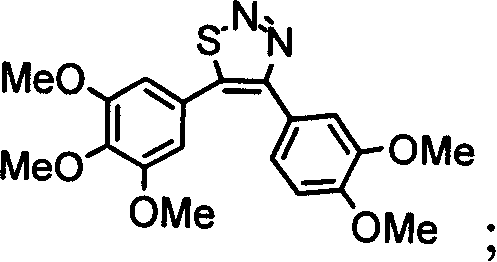
4,5-di-substituted-phenyl-1,2,3-thiadia-zole derivative, and its preparation method and uses
A technology for thiadiazoles and derivatives, applied in 4 fields, can solve the problems of complex synthesis, poor water solubility, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Preparation of 4-p-methoxyphenyl-5-(3,4,5-trimethoxyphenyl)-1,2,3-thiadiazole (compound 15) represented by the following structural formula.
[0068]
[0069] Dissolve 2-(3,4,5-trimethoxyphenyl)-1-(4-methoxyphenyl)-ethanone (1mmol) and p-toluenesulfonylhydrazide (2mmol) in ethanol (25ml) , heating to reflux for 3h, distilling off the solvent under reduced pressure, and draining with an oil pump. Dissolve in dry chloroform (30 ml), add thionyl chloride (2 ml) carefully dropwise, cool with ice water, naturally rise to room temperature, and react for 4 hours. Stop the reaction, evaporate the solvent under reduced pressure, add saturated aqueous sodium bicarbonate solution, separate the layers, and extract. The organic phase was washed successively with saturated aqueous sodium bicarbonate solution and saturated sodium chloride solution, and dried over anhydrous sodium sulfate. The product was obtained by column separation with a yield of 51%.
[0070]Mp: 9...
Embodiment 2
[0071] Embodiment 2: the preparation of compound 1
[0072] Compound 1 was prepared in the same manner as in Example 1 except that corresponding raw materials were used. The structural formula, melting point, and NMR and MS data of Compound 1 are listed in Table 1 below.
Embodiment 3
[0073] Embodiment 3: the preparation of compound 2
[0074] Compound 2 was prepared in the same manner as in Example 1 except that corresponding raw materials were used. The structural formula, melting point, and NMR and MS data of Compound 2 are listed in Table 1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com