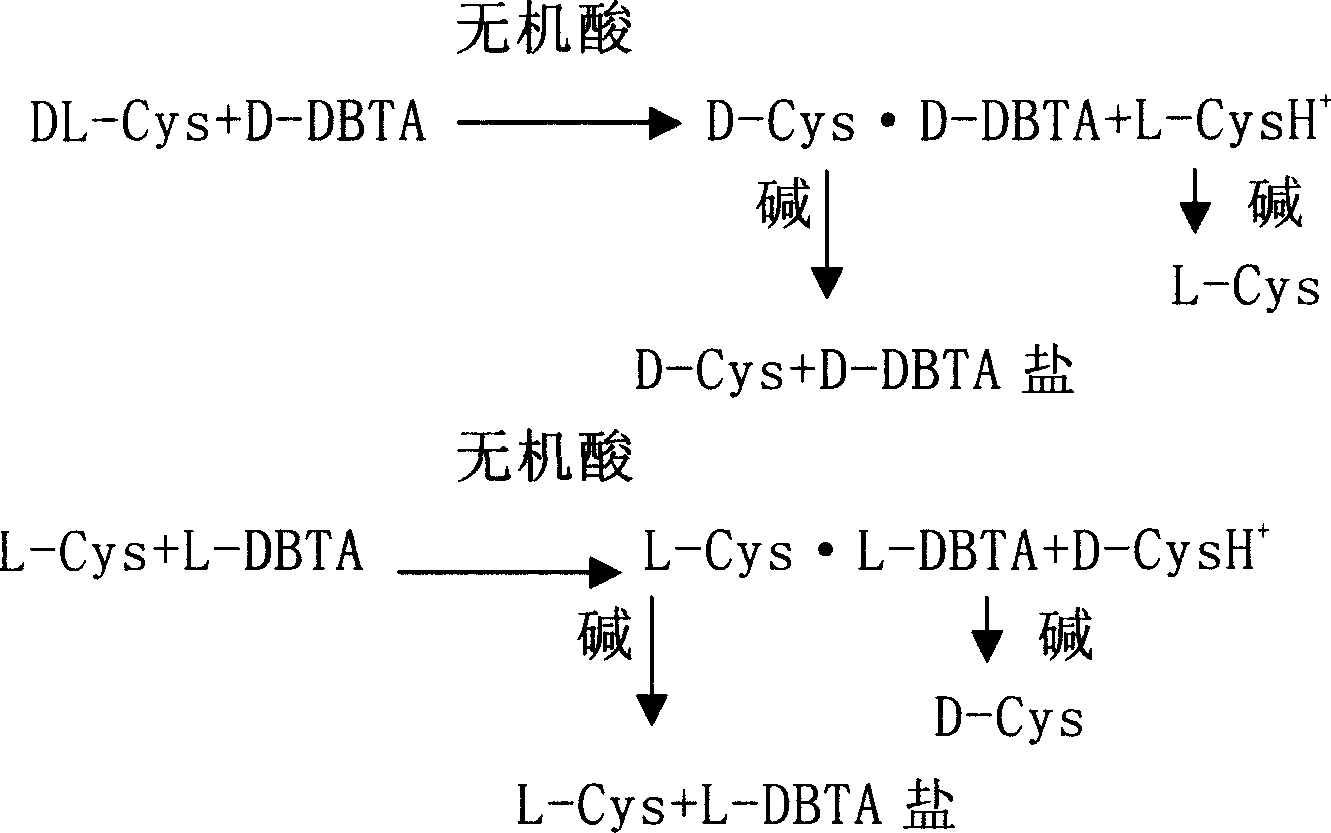
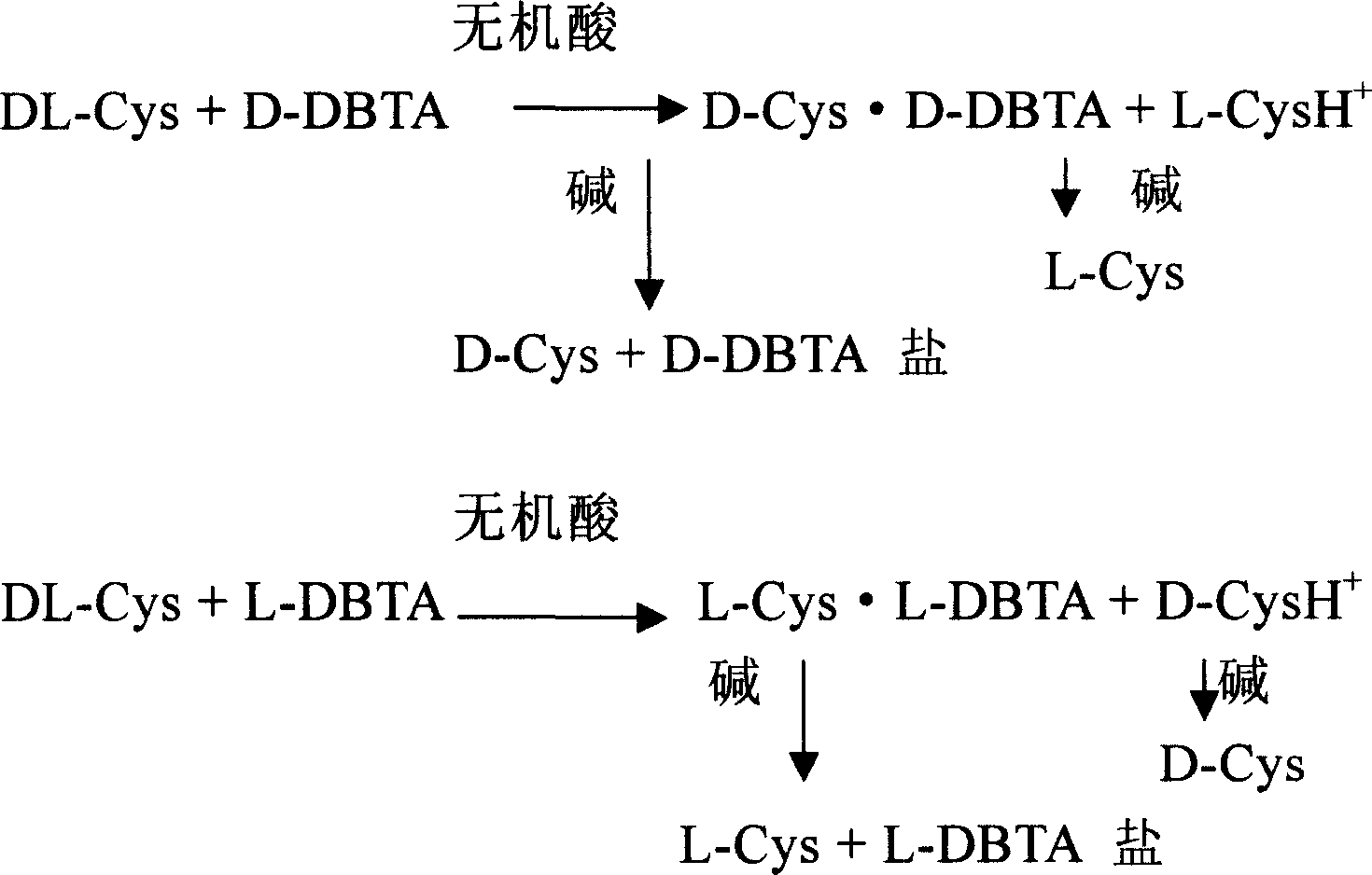
Method for preparing levocysteine and dextral cysteine using chemical resolution method
A technology of L-cysteine and cysteine, which is applied in the field of preparation of L-cysteine and D-cysteine, can solve the problems of low product yield and the like, and achieves simple synthesis process and little environmental pollution. , the effect of reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] DL-Cys2.43g (0.02mol) was dissolved in 20ml of dilute hydrochloric acid, heated to 80-100°C under stirring, added L-DBTA3.58g (0.01mol), reacted at this temperature for half an hour, and then kept at 60°C After 1 hour, a precipitate gradually formed, then cooled to about 15°C, filtered with suction, dissolved the filter cake in 30ml of ethanol and 2 times the amount of triethylamine, stirred for 1 hour, filtered with suction, washed with ethanol, and dried to obtain 1.06g L -Cys, resolution yield 87.5%, [α] D 20=+8.7° (C=8, 1N HCl), the optical purity of L-Cys product is 99.3%. Split and concentrate the mother liquor, adjust the pH value to 5.5 with alkali, stir, a white solid precipitates out, filter with suction and wash with ethanol, dry to obtain 0.96g of D-Cys, the yield is 79.2%, [α] D 22 =-8.6° (C=8, 1N HCl), the optical purity of the D-Cys product is 99.2%.
Embodiment 2
[0040] DL-Cys2.43g (0.02mol) was dissolved in 20ml dilute hydrochloric acid, stirred, heated to 90-100°C, added 3.58g D-DBTA, reacted at this temperature for 50 minutes, then kept at 60°C for 1 hour, gradually precipitated formed, then cooled to about 15°C, filtered with suction and washed with water, dissolved the filter cake in 30ml of ethanol and 2 times the amount of triethylamine, stirred for 1 hour, filtered with suction, washed with ethanol, and dried to obtain 1.06g of D-Cys. Resolution yield 87.5%, [α] D 22 =-8.8° (C=8, 1N HCl), the optical purity of the D-Cys product is 99.5%. . Split and concentrate the mother liquor, adjust the pH value to 5.5 with alkali, stir, a white solid precipitates, filter with suction and wash with ethanol, dry to obtain 0.94g L-Cys, yield 77.4%, [α] D 22 =+8.7° (C=8, 1N HCl), the optical purity of the D-Cys product is 99.3%.
Embodiment 3
[0042] DL-Cys2.43g (0.02mol) was dissolved in 20ml of dilute hydrochloric acid, heated to 80-100°C under stirring, added L-DBTA7.16g (0.02mol), reacted at this temperature for half an hour, and then kept at 60°C After 1 hour, a precipitate gradually formed, then cooled to about 15°C, filtered with suction, dissolved the filter cake in 30ml of ethanol and 2 times the amount of triethylamine, stirred for 1 hour, filtered with suction, washed with ethanol, and dried to obtain 1.08g L -Cys, resolution yield 88.9%, [α] D 20 =+8.8° (C=8, 1N HCl), the optical purity of L-Cys product is 99.5%. Split and concentrate the mother liquor, adjust the pH value to 5.5 with alkali, stir, a white solid precipitates out, filter with suction and wash with ethanol, dry to obtain 0.90 g of D-Cys, the yield is 74.1%, [α] D 22 =-8.6° (C=8, 1N HCl), the optical purity of the D-Cys product is 99.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com