Clean production method for dioctyl phthalate
A technology for clean production of dioctyl phthalate, applied in the field of clean production of dioctyl phthalate, can solve the problems of high material consumption and energy consumption, long production process, large investment in equipment, etc., and achieve pollutant discharge Less water consumption, lower water consumption, and higher product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
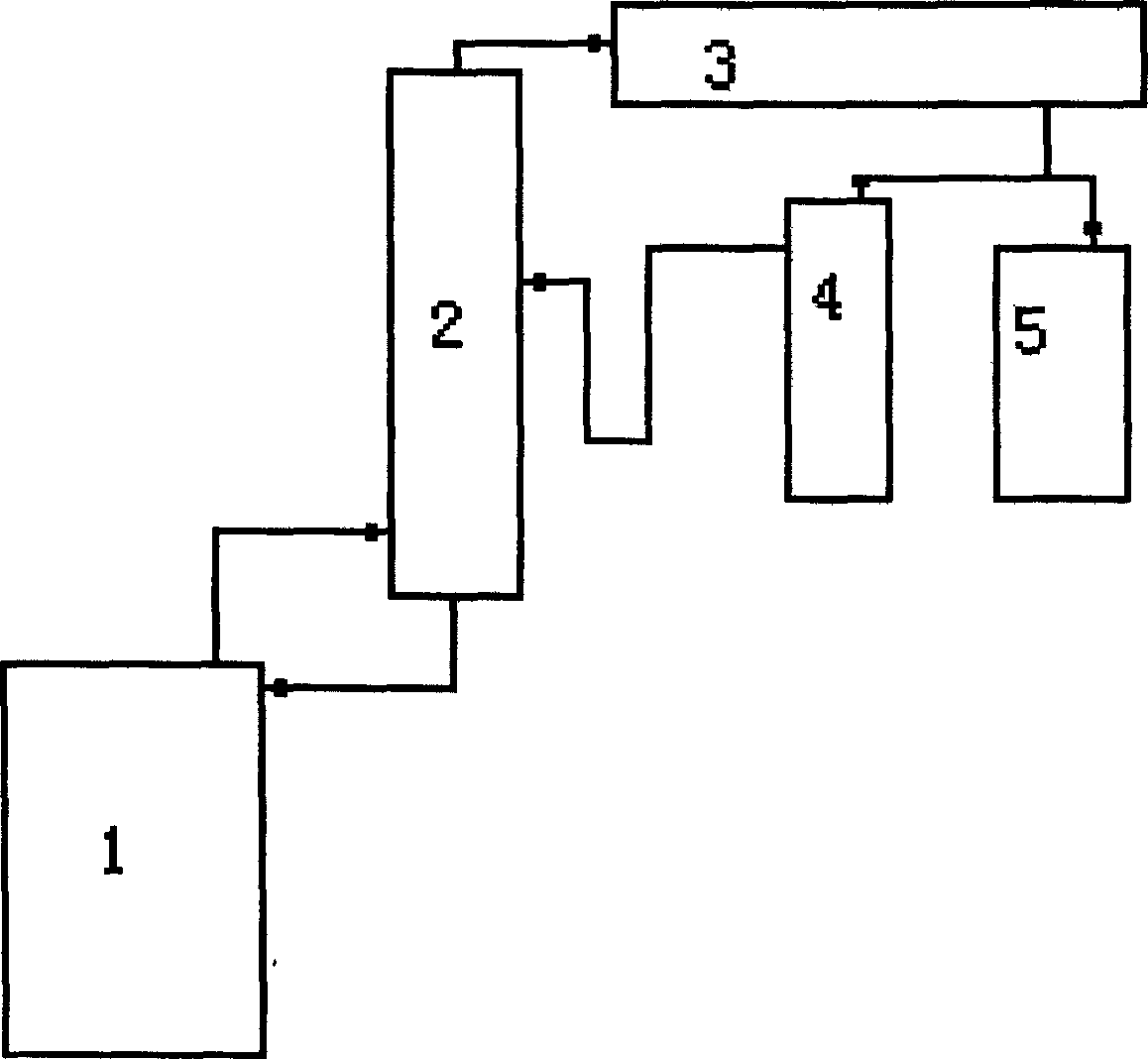
[0021] As shown in the process flow diagram of the present invention shown in Figure 1, 1000g of phthalic anhydride and 3000g of octanol are used as raw materials in a percentage by weight of 1: 2 to 3, and tetraisopropyl titanate is used as a catalyst, and the addition amount is 6g of the total material. In the reaction kettle 1, the esterification reaction is carried out by heating under normal pressure. The excess octanol and the water generated by the esterification reaction form an alcohol-water azeotrope, which is separated by the esterification tower 2, and part of the alcohol is returned to the reaction kettle. After the esterification condenser 3 is condensed and cooled, it is collected into the phase separator 4, and after settling and separation, the alcohol is returned to the esterification tower, and the reaction end point temperature is 220°C for complete reaction; Steam deoctanol, this process can remove 60% excess octanol in the esterification reaction, collect ...
Embodiment 2
[0023] Same as Example 1, except: 1,500 g of phthalic anhydride, 2,000 g of octanol and 1,000 g of recovered alcohol were used as raw materials, tetraisopropyl titanate was used as a catalyst, the addition amount was 28 g of the total material, and the reaction end point temperature was 230° C. for complete reaction; During the esterification reaction, 70% of excess octanol was removed, and the temperature in the kettle dropped to 180° C. after flash evaporation.
Embodiment 3
[0025] Same as Example 1, the difference is: 1000g of phthalic anhydride and 2500g of octanol as raw materials, using tetraisopropyl titanate as catalyst, the addition amount is 18g of the total material, and the reaction terminal temperature is 240°C for complete reaction; 80 % excess octanol is released, and the temperature in the kettle drops to 190° C. after flashing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com