Adiabatic-isothermal shift technique for high-concentration CO raw gas
A technology of isothermal transformation and raw gas, applied in the fields of inorganic chemistry, non-metallic elements, chemical instruments and methods, etc., can solve the problems of process flow setting and control method differences, etc., to reduce one-time usage, reduce system resistance, and save The effect of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
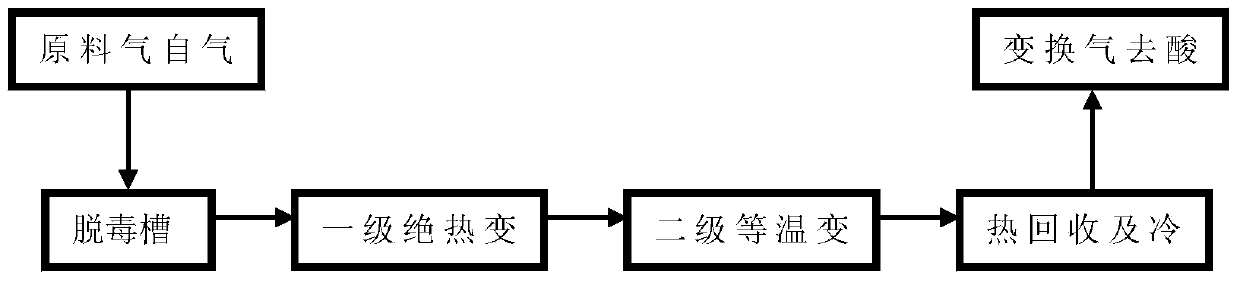
[0036] "Adiabatic + isothermal" two-stage sulfur conversion
[0037] The raw material gas with a CO dry basis volume content of 70% and a water-gas ratio of 1.1 first enters the detoxification tank to remove dust and other impurities, and then exchanges heat with the gas after the first-stage adiabatic shift reaction to 240°C and enters the first reactor Carry out one-stage shift reaction, the hot spot temperature of the first-stage reactor bed layer is 400°C, the reaction pressure is 3.6MPa, and the dry basis content of CO in the outlet gas of the first-stage reactor is 30%; the gas after the first-stage adiabatic shift is firstly mixed with The inlet raw material gas of the first-stage reactor is heat-exchanged, and then passes through the self-produced medium-pressure saturated steam of a steam superheater superheating system, and then recovers heat through the waste pot, adjusts the temperature to 240°C, and enters the second-stage isothermal shift reactor Continue the con...
example 1
[0039] The "adiabatic + isothermal" two-stage sulfur-resistant shift production process described in Example 1, wherein a layered temperature-controlled reactor is used for the first-stage shift reaction, and a cobalt-molybdenum-based sulfur-tolerant shift catalyst is used in the CO shift reaction process, and the qualified The shift gas can be used in synthetic ammonia and hydrogen production units.
Embodiment 2
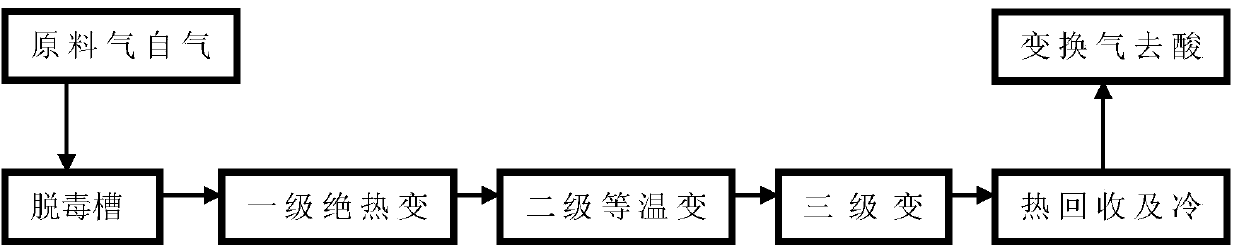
[0041] "Adiabatic + isothermal + adiabatic" three-stage sulfur conversion
[0042] The raw material gas with a CO dry basis content of 70% and a water-gas ratio of 0.76 first enters the detoxification tank to remove impurities such as dust, and then exchanges heat with the gas after the first-stage adiabatic shift reaction to 240°C, and then enters the first reactor for further processing. First-stage shift reaction, the hot spot temperature of the first-stage reactor bed is 350°C, the reaction pressure is 3.6MPa, and the dry content of CO in the gas at the outlet of the first-stage reactor is 40%; The inlet raw material gas of the primary reactor is heat-exchanged, and then passes through the medium-pressure saturated steam produced by the superheating system of a steam superheater, and then superheated steam and quenching water are added, and the temperature is adjusted to 250°C to enter the second-stage isothermal transformation reaction The reactor continues to shift react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com