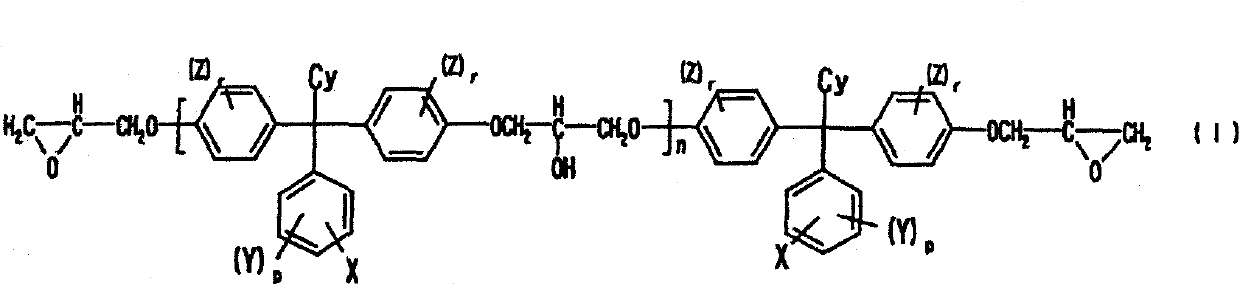
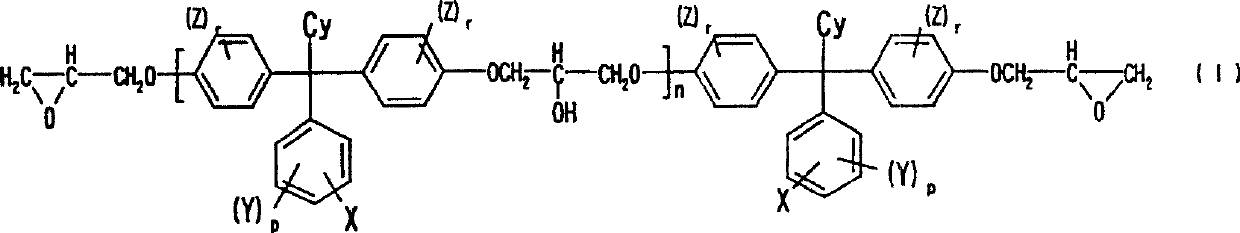
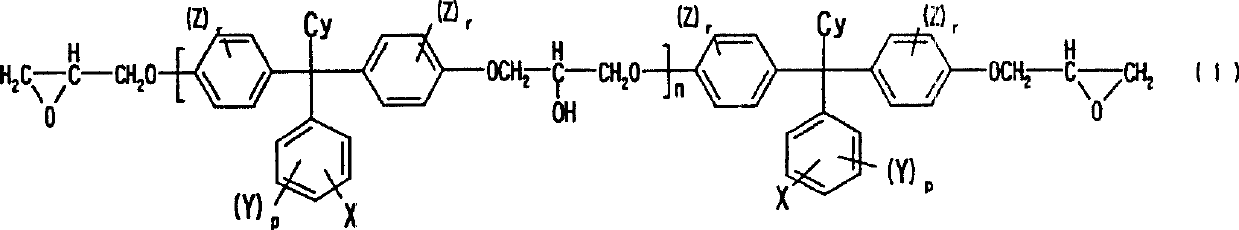
Alkali developing photosensitive resin composition
A technology of resin composition and developability, applied in optics, photography, opto-mechanical equipment, etc., can solve the problems of insufficient sensitivity of photosensitive resin composition, difficulty in obtaining graphic shapes and fine patterns, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] [Example 1] Production of Alkaline Developable Resin Composition No.1
[0095] Production of 1,1-bis(4'-hydroxyphenyl)-1-(1"-biphenyl)-1-cyclohexylmethane
[0096] 70.5 g of biphenylcyclohexyl ketone, 200.7 g of phenol, and 10.15 g of thioacetic acid were added, and 40.0 g of trifluoromethanesulfonic acid was added dropwise at 18° C. over 20 minutes. After reacting at 17-19° C. for 18 hours, 500 g of water was added to stop the reaction, 500 g of toluene was added, the organic layer was washed with water until its pH reached 3-4, and the organic layer was separated. Toluene, water and excess phenol were distilled off. Toluene was added to the residue, and the precipitated solid was filtered, dispersed and washed with toluene to obtain 59.2 g of pale yellow crystals (51% yield). The melting point of the pale yellow crystals was 239.5° C., and it was confirmed that the pale yellow crystals were the target compound.
[0097] Production of 1,1-bis(4'-glycidoxyphenyl)-1...
Embodiment 2
[0102] [Example 2] Production of Alkaline Developable Resin Composition No.2
[0103] Add 43g1,1-bis(4'-glycidoxy phenyl)-1-(1"-biphenyl)-1-cyclohexylmethane (compound a), 33.6g acrylic acid (compound b-1), 0.04g2,6-di-tert-butyl-p-cresol, 0.21g tetrabutylammonium acetate and 18g propylene glycol-1-monomethyl ether-2-acetate were stirred at 120°C for 13 hours. Cooled to room temperature, Add 24g propylene glycol-1-monomethyl ether-2-acetate and 10g succinic anhydride (compound c-1), and stir for 3 hours at 100°C. Then add 8g bisphenol Z glycidyl ether (compound d-2), After stirring at 120°C for 4 hours, at 90°C for 3 hours, at 60°C for 2 hours, and at 40°C for 5 hours, add 29g of propylene glycol-1-monomethyl ether-2-acetate to obtain propylene glycol- Alkaline developable resin composition No. 2 (Mw=4600, Mn=2100, acid value (solid content) 54 mgKOH / g) of the target product in the form of 1-monomethyl ether-2-acetate solution.
[0104] In addition, the reaction product cont...
Embodiment 3
[0105] [Example 3] Production of Alkaline Developable Resin Composition No.3
[0106]Add 1695g1, 1-bis(4'-epoxypropoxyphenyl)-1-(1"-biphenyl)-1-cyclohexylmethane (compound a), 443g acrylic acid (compound b-1), 6g2 , 6-di-tert-butyl-p-cresol, 11g tetrabutylammonium acetate and 1425g propylene glycol-1-monomethyl ether-2-acetate, stirred at 120°C for 16 hours. Cooled to room temperature, added 931g propylene glycol -1-monomethyl ether-2-acetate, 741 g of hexahydrophthalic anhydride (hereinafter also referred to as compound c-2) and 25 g of tetra-n-butylammonium acetate were stirred at 70° C. for 4 hours. Then add 313g ethylene glycol diglycidyl ether (hereinafter also referred to as compound d-3) and 1463g propylene glycol-1-monomethyl ether-2-acetate, thereby obtain as propylene glycol-1-monomethyl ether-2-ethyl ether Alkali-developable resin composition No. 3 (Mw = 3000, Mn = 1700, acid value (solid content) 43 mgKOH / g) of the target product in the form of an acid acid ester ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com