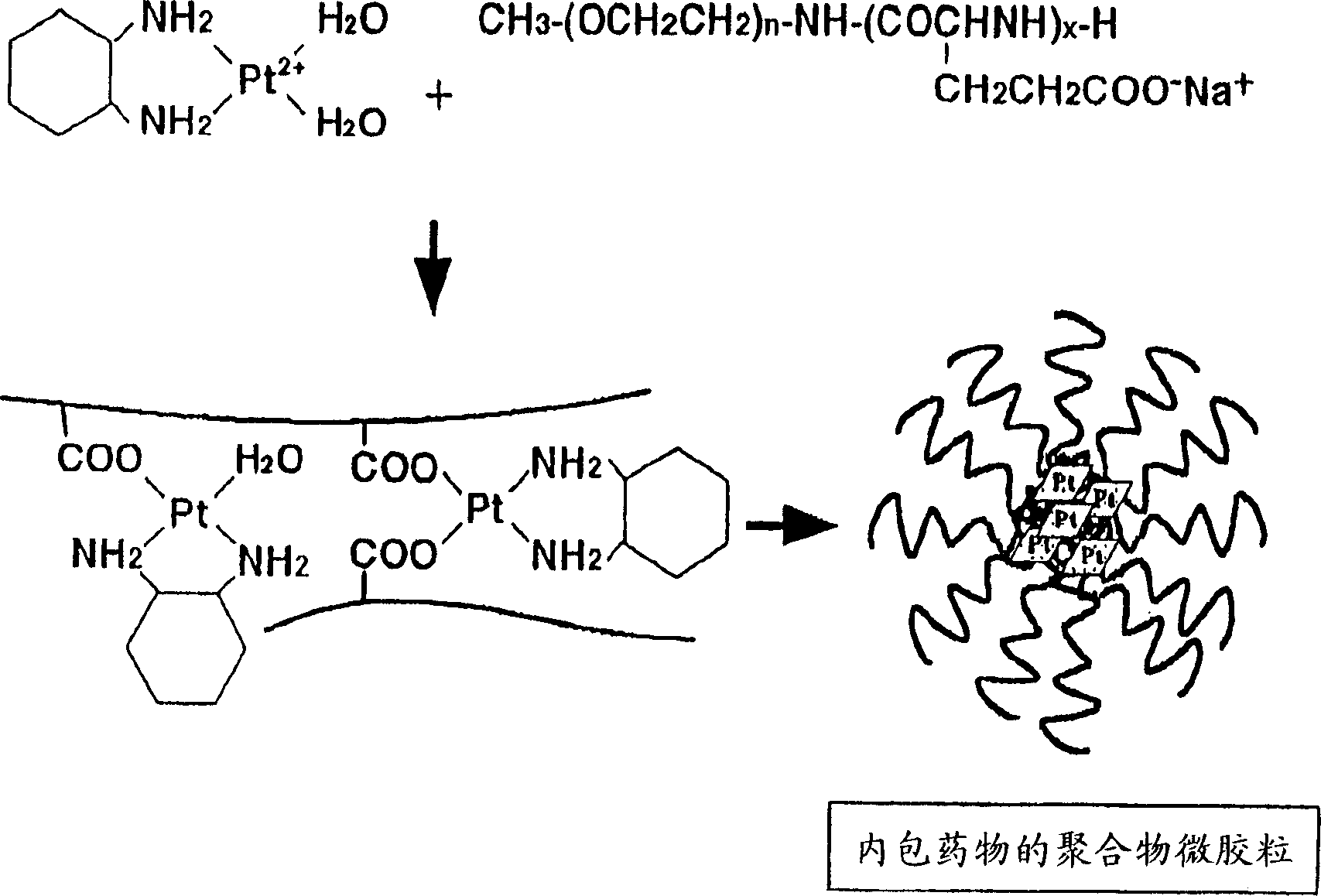
Coordination complex of diaminocyclohexaneplatinum(II) with block copolymer containing poly(carboxylic acid) segment and antitumor agent comprising the same
A technology of diaminocyclohexane and block copolymers, which can be used in antitumor drugs, platinum-based organic compounds, platinum-group organic compounds, etc. It can solve the problems of high cytotoxicity and achieve high anti-tumor efficacy and excellent stability , the effect of safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Generation of polymer micelles
[0067] The structural formula of the block copolymer used in this example is shown below.
[0068]
[0069] (1.a.) Suspend 19 mg of dichloro(1,2-diaminocyclohexane) platinum(II) (dichloro-DACHPt) in 10 ml of distilled water and mix with 12.3 mg of silver nitrate ([AgNO 3 ] / [dichloro-DACHPt] molar ratio = 1.5). The mixture was kept in the dark at 25°C for 24 hours. After the reaction, silver chloride precipitated out. Next, the reaction mixture was centrifuged at 3000 rpm for 5 minutes to remove the precipitated silver chloride. The Diaquo DACHPt-containing supernatant thus obtained was purified through a 0.22 μm filter.
[0070] (1.b.) 24.4 mg of the block copolymer represented by the above structural formula (hereinafter referred to as PEG-P(Glu) 12-35) was added to 10 ml of the purified supernatant obtained in the above (1.a.) ( The molar ratio of "Diaquo-DACHPt" / [Glu] = 1), wherein the above-mentioned block copolyme...
Embodiment 2
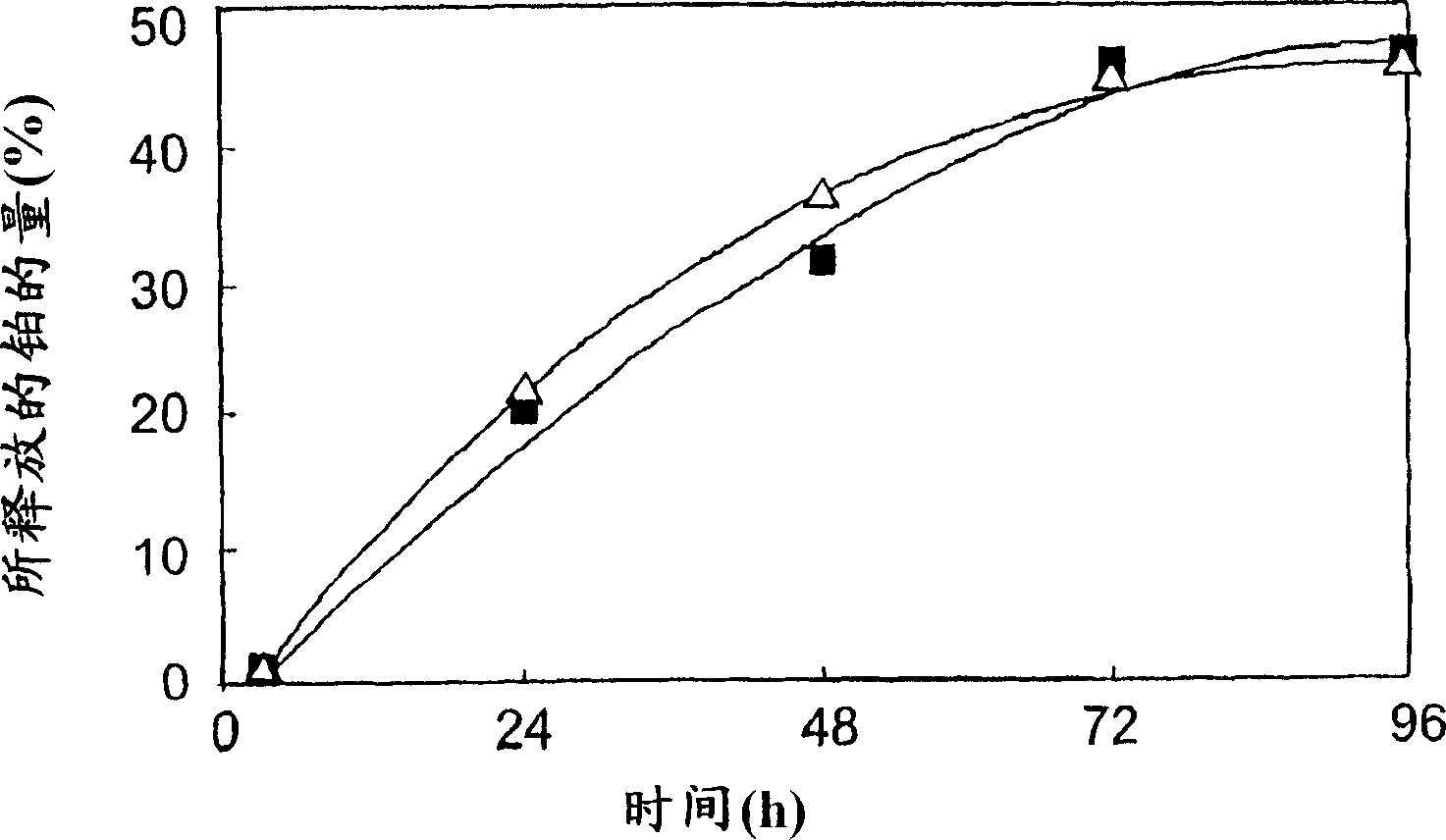
[0072] Example 2: Properties of polymer micelles
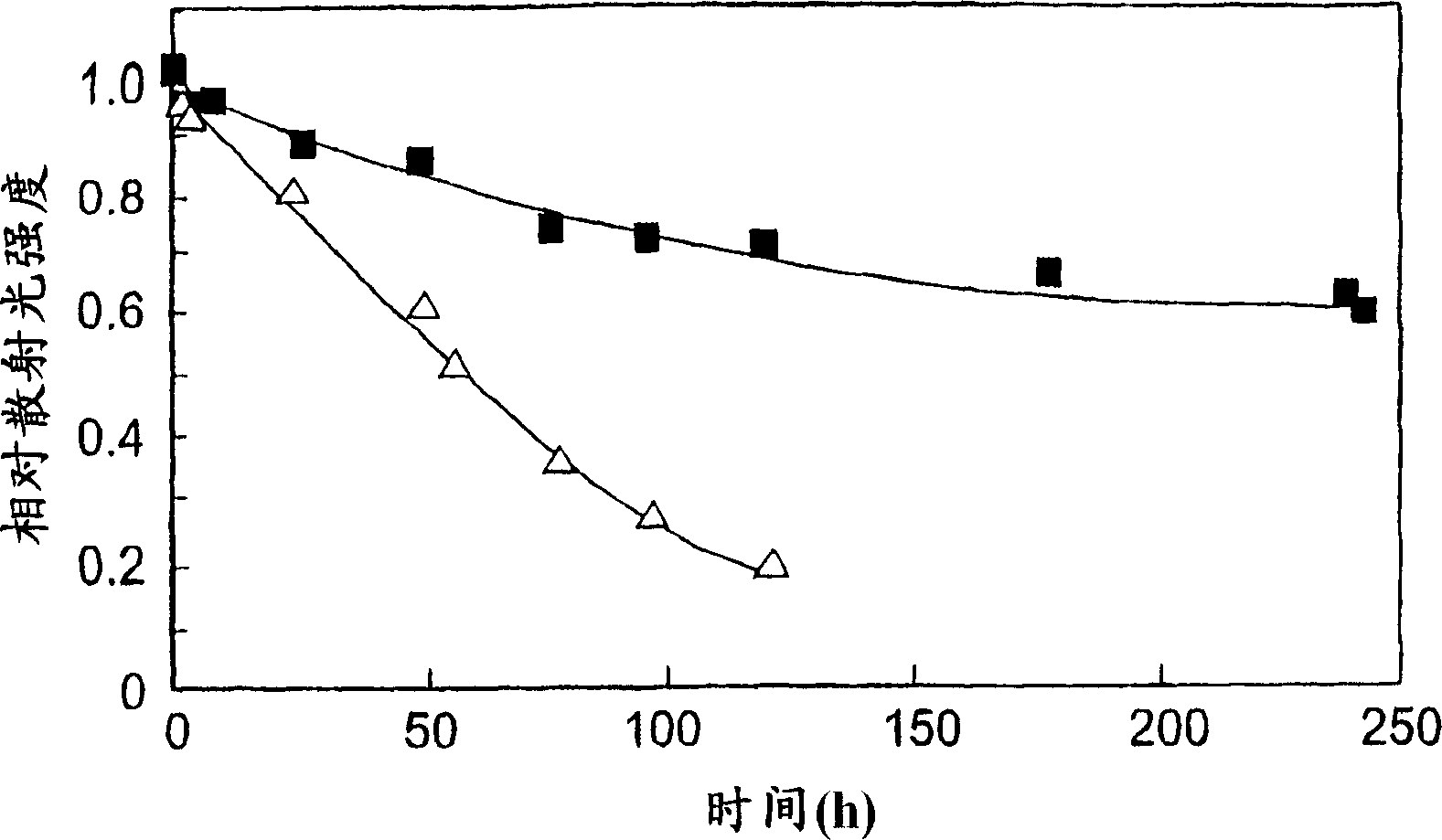
[0073] (2.a.) Stability in 10mM PBS
[0074] Using the same block copolymer as used above, the stability of the polymer micelles obtained according to Example 1 (hereinafter referred to as DACHPt micelles) was compared with that of the inner capsule produced as described in Example 1 of the above-mentioned WO02 / 26241A1. Stability of polymer micelles of cisplatin (hereinafter referred to as CDDP micelles).
[0075] CDDP micelles were prepared briefly as follows:
[0076] Cisplatin (CDDP) was dissolved in water (5 mmol / l). The same block copolymer as used in Example 1 above was dissolved in this solution ([CDDP] / [Glu of copolymer]=1.0). It was allowed to react at 37°C for 73 hours. The resulting solution was repeatedly subjected to ultrafiltration (MWCO=100,000) to be purified. The formation of polymer micelles was confirmed by measuring dynamic light scattering (DLS).
[0077] First, the micelle concentration is determine...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap