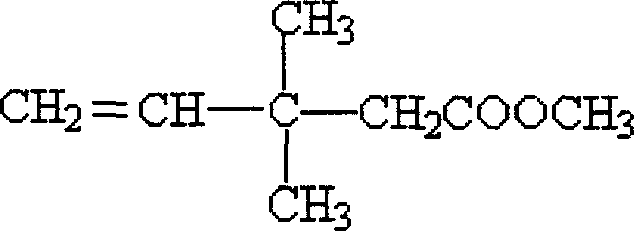
Method of preparing 3,3-dimethyl-4-pentenoic acid methyl ester with industrial scale
An industrial-scale technology of methyl bentitate, which is applied in the field of carbocyclic compound preparation, can solve the problems of no solid catalyst and pressurized reaction, and achieve the effects of less "three wastes" emission, simple process, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
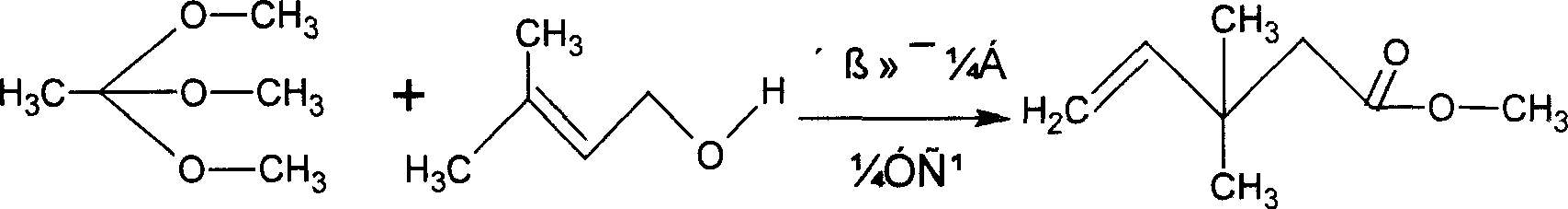
[0023] Molar ratio: isopentenol: trimethyl orthoacetate: catalyst = 1: 1.2: 0.05
[0024] Raw materials: (all adopt commercially available industrial specifications)
[0025] Commercially available prenyl alcohol (Prenyl alcohol, chemical name 3-methyl-2-buten-1-ol 3-methyl-2-buten-1-ol) 86.9kg (content 99%, pure 86.031kg 1gkmol )
[0026] Commercially available trimethyl orthoacetate (Trimethyl orthoacetate, chemical name: 1.1.1-trimethoxyethane, 1.1.1-Trimethoxyethane) 145.5kg (content 99%, pure 144.05kg 1.2kgmol)
[0027] Catalyst anhydrous zinc chloride 6.8kg (content 98%, pure 6.664kg, 0.05kgmol)
[0028] operate:
[0029] Put the raw materials and catalysts into the autoclave, heat, and react for 6-7 hours at 150-160°C and a pressure of 2.5Mpa in the autoclave; after the reaction is completed, cool down to room temperature, and the pressure in the autoclave will naturally drop to normal pressure; the vacuum sucks out the material, and the reaction material is filtered...
Embodiment 2
[0031] Molar ratio: isopentenol: trimethyl orthoacetate: catalyst = 1: 1.5: 0.03
[0032] Prenol 86.9kg (content 99%, pure 86.031kg 1kgmol) and trimethyl orthoacetate 181.8kg (content 99%, pure 179.98kg 1.5kgmol), catalyst nickel sulfate 7.7kg (content 98%, pure 7.55kg, 0.03kgmol) in an autoclave at 190-200°C, 3.0MPa, react for 8-9h, and observe that the pressure no longer increases as the end point of the reaction. The rectification operation was the same as that in Example 1, and finally the product methyl bentinate was 126kg, and the content determined by gas chromatography was 99.26%, which was 125.07kg pure, and the yield was 88.08%.
Embodiment 3
[0034] Molar ratio: isopentenol: trimethyl orthoacetate: catalyst = 1: 1.2: 0.02
[0035] Prenol 86.9kg (content 99%, pure 86kg 1kgmol) and trimethyl orthoacetate 145.5kg (content 99%, pure 144.05kg 1.2kgmol), catalyst nickel acetate 5.3kg (content 98%, pure 5.19 kg, 0.02kgmol) into the autoclave, 160~170°C, 2.5MPa, react for 9~10h, observe the end of the reaction after the pressure no longer increases. The rectification operation was the same as that in Example 1, and finally the product methyl bentinate was 122kg, and the content determined by gas chromatography was 99.43%, which was 121.31kg pure, and the yield was 85.43%. All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com