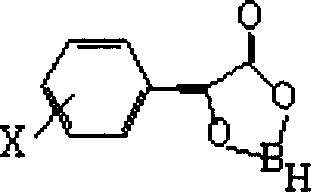
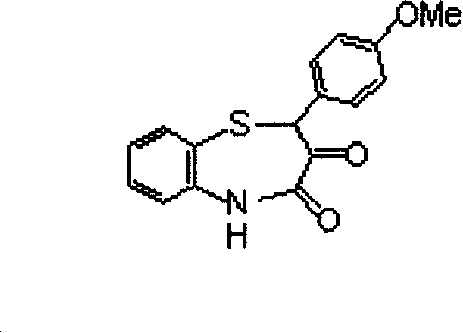
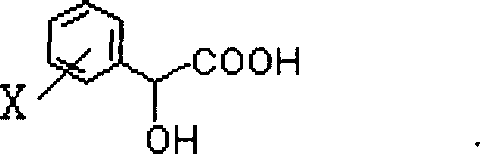Process for preparing diltiazem intermediate
A technology for diltiazem and intermediates, applied in the field of preparation of diltiazem intermediates, can solve the problems of being difficult to use, difficult to obtain, etc., and achieve the effects of easy industrial production and high optical yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 125 ml Erlenmeyer flask with a stirring reflux device, under the protection of nitrogen, add 100 ml of anhydrous tetrahydrofuran solvent, 0.457 g (0.012 mol) of NaBH 4 and 2.6 g (0.016 mol) of optically active R-mandelic acid ([α] 25 D =-150°, c=2, water), NaBH 4 The molar ratio with the optically active R-type mandelic acid derivative is 1: 1.3, heat and reflux for 3 hours, then cool the reaction solution with ice-salt water, add 3 grams (0.01 mole) of diltiazem intermediate I at 0 ° C, optical The mol ratio of active R-type mandelic acid and diltiazem intermediate I is 1: 0.62, and the solution is continued to be cooled to -10~-20 DEG C, kept stirring at this temperature for 3 hours, then stop the reaction with a small amount of room temperature water, reduce Add 100 milliliters of water after removing tetrahydrofuran by pressure distillation, stir for 0.5 hour, make the product become powdery solid in water at room temperature, vacuum filter the precipitated s...
Embodiment 2
[0028] 1 g of the crude product of diltiazem intermediate II obtained in Example 1 was recrystallized with 40 ml of absolute ethanol to obtain 0.88 g of white crystals of diltiazem intermediate II with a yield of 88%, m.p.171°C to 177°C , [α] 23 D =+74.3° (c=0.3, absolute ethanol), chiral HPLC analysis: (2R, 3R) isomer is 0.9%, (2S, 3S) isomer is 99.1%.
Embodiment 3
[0030] In a dry 125 ml three-necked flask with stirring reflux, under N 2 Under protection, add 100 ml of anhydrous tetrahydrofuran, 0.609 g (0.016 mol) of NaBH 4 , 6.5 g (0.04 mol) of R-mandelic acid, NaBH 4 The molar ratio with the optically active R-type mandelic acid derivative is 1: 2.5, heat and reflux for 3 hours, then cool the reaction solution with ice-salt water, add 9.6g (0.032mol) diltiazem intermediate I at 0°C, the optically active The mol ratio of R-type mandelic acid and diltiazem intermediate is 1: 0.8, and continue to cool the solution to -10~-20°C, keep stirring at this temperature for 3 hours, then stop the reaction with a small amount of water at room temperature, and The tetrahydrofuran was removed, and 100 ml of water was added to the residue. Stir for 0.5 hours to make the product a powdery solid in water at room temperature, vacuum filter the precipitated solid, and dry to obtain diltiazem intermediate II, namely d-cis-3-(4-methoxyphenyl)-3-hydroxyl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com