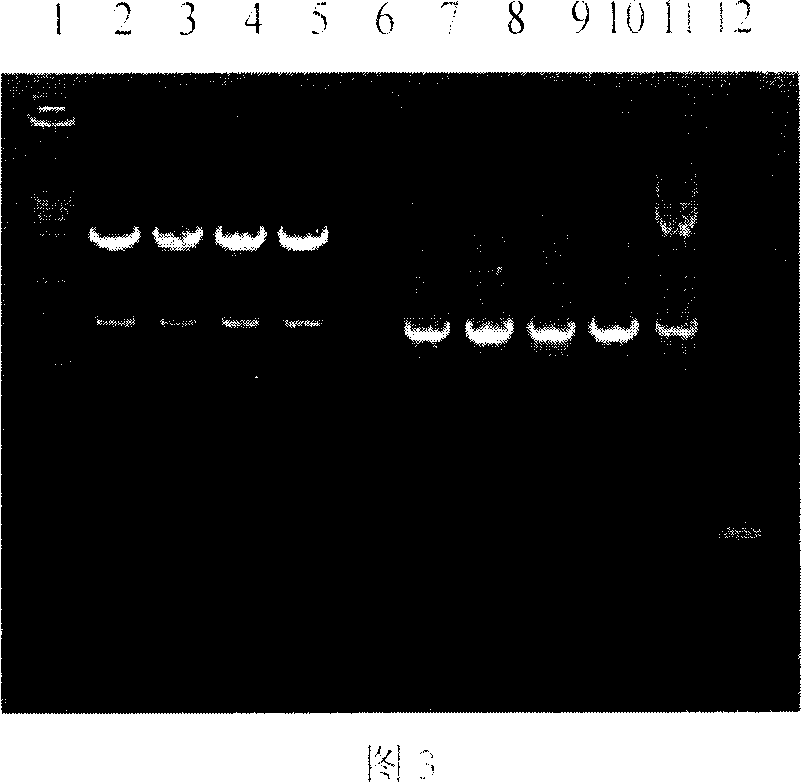Combination immunizing preparation comprising recombinant plasmid and duplicate deficit type recombinant adenovirus
A replication-deficient, recombinant adenovirus technology, applied in the field of combined immunization preparations, which can solve problems such as non-specific damage, wandering, and treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
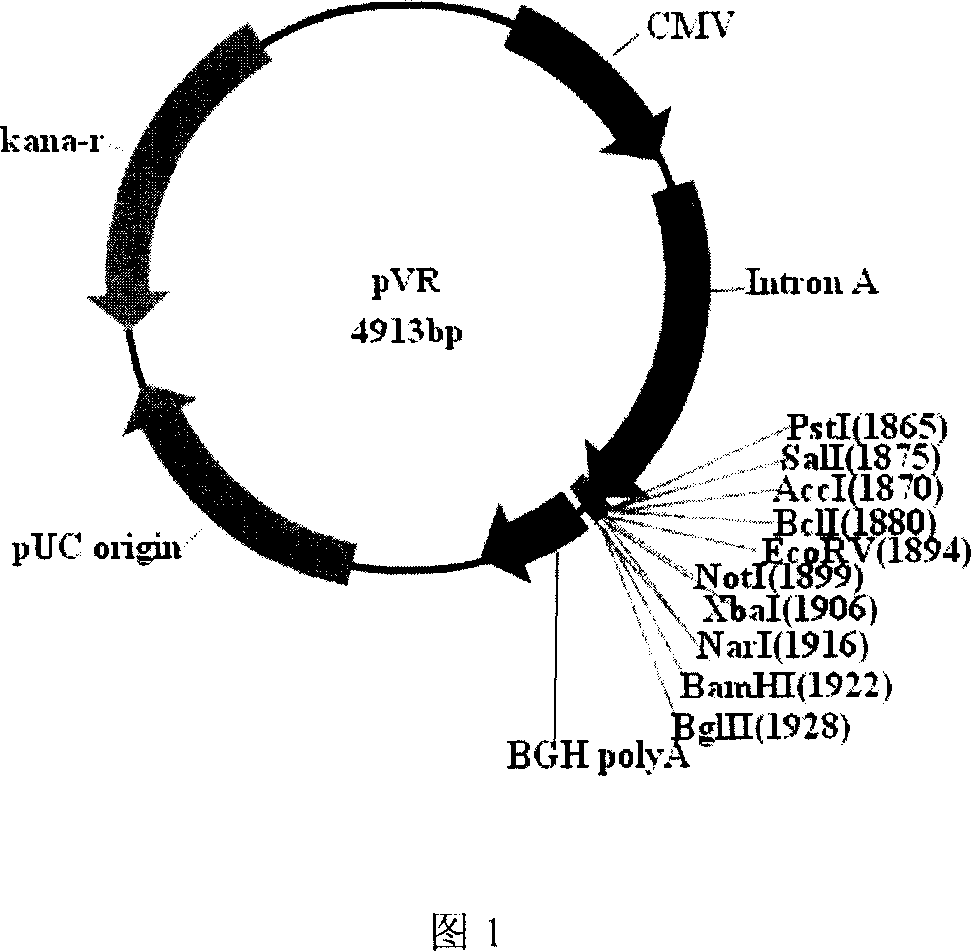
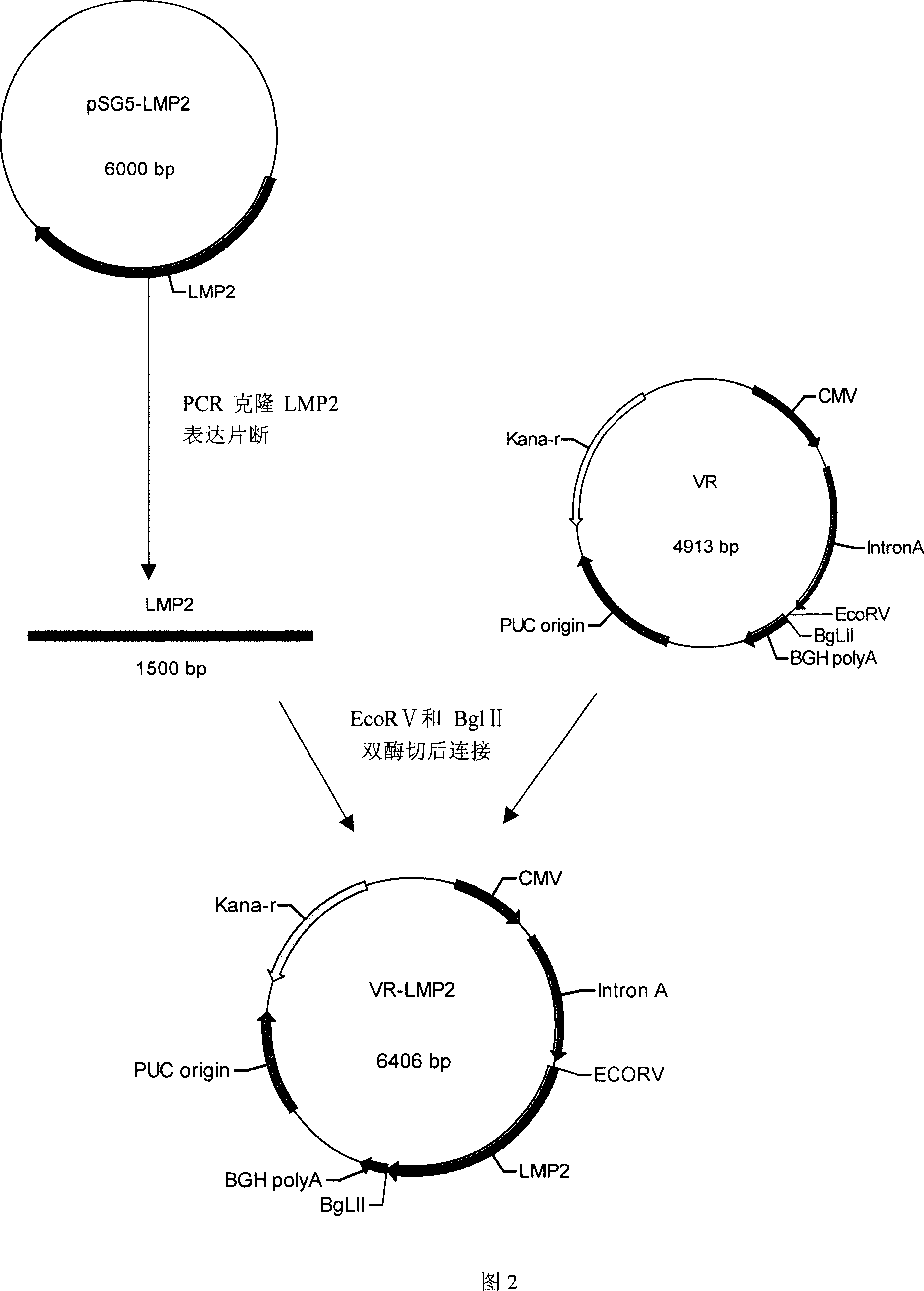
[0046] Example 1 Construction and Identification of Recombinant Plasmid pVR-lmp2 Containing LMP2 Sequence
[0047] In this example, the recombinant plasmid pVR-LMP2 containing the LMP2 sequence was constructed (see Figure 2 for the construction process), and the constructed recombinant plasmid was identified by recombinant plasmid sequencing and recombinant protein expression.
[0048] 1.1 Preparation of Escherichia coli DH5α competent cells
[0049] Pick a single colony of DH5α from a fresh plate cultured at 37°C for 16-20 hours, inoculate it in 5 ml of LB medium without antibiotics, and culture it overnight (12-16 hours) at 37°C with vigorous shaking. On the next day, draw 0.5ml from the above-mentioned culture and transfer it to 50ml LB medium at a ratio of 1:100 to continue culturing for about 3 hours until the OD600 value of the bacterial solution is 3. , In an ice-precooled 50ml centrifuge tube, ice bath for 30min. Centrifuge at 4000rpm for 10min at 4°C, discard the su...
Embodiment 2
[0084] Construction of Ad5 Replication Deficient Recombinant Adenovirus Containing LMP2 Sequence
[0085] In this example, a replication-defective recombinant adenovirus Ad5-LMP2 containing the LMP2 sequence was constructed.
[0086] 2.1 Construction of Ad5 replication-deficient recombinant adenoviral shuttle plasmid with LMP2 sequence
[0087] (1) Transformation of PSG5-LMP2 plasmid:
[0088] Pipette 1 μl PSG5-LMP2 plasmid with a sterile tip and add it to 200 μl DH5α competent cells, swirl gently to mix well, and place in ice bath for 30 minutes. Move the tube into a water bath at 42°C for 90 seconds, then quickly transfer the tube to an ice bath to cool the cells for 1-2 minutes. Add 800 μl of LB medium without antibiotics to each tube, move to a shaker at 37° C., and incubate for 45 minutes (rotational speed <150 rpm). Take 50 μl of transformed competent cells, use a sterile elbow glass rod to lightly coat the surface of the agar plate containing the correspo...
Embodiment 3
[0113] Identification, viral titer analysis and protein expression of a replication-deficient recombinant adenovirus Ad5-LMP2
[0114] 3.1 Transcription of LMP2 gene in 293 cells infected with recombinant adenovirus
[0115] TRIzol one-step method was used to extract the total RNA of normal 293 cells, Ad5-infected 293 cells and Ad5-LMP2-infected 293 cells for RT-PCR identification. RT-PCR reaction parameters are as follows: reverse transcription system is 20 μl. Use 1 μg of total cellular RNA as a template, Oligod(T)15 as a primer, perform reverse transcription reaction at 50°C for 30 minutes, inactivate reverse transcriptase at 94°C for 2 minutes, and perform PCR reaction with synthesized cDNA as a template, upstream primer P1: 5′GCTGCAGGAAACAACTCCCAATATCCA 3'; downstream primer P2: 5'AACTGGAGGGCAGCATCTAATGACC 3'. 30 cycles of 95°C for 30s, 55°C for 45s, and 72°C for 60s, 5 μl of RT-PCR reaction products were taken for agarose gel electrophoresis analysis. The results show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com