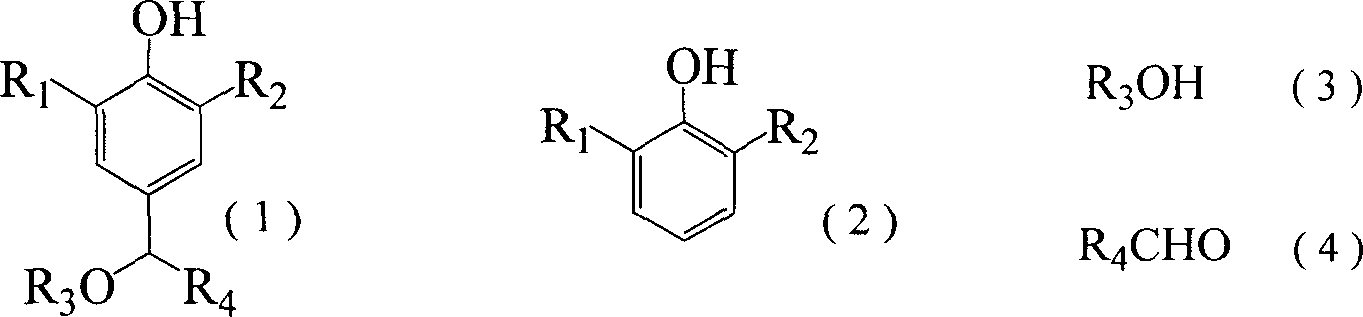
Synthesis of alkoxy alkyl substituted phenol at normal pressure
A technology for synthesizing alkoxyalkyl groups and alkoxyalkyl groups, which is applied in ether preparation, organic chemistry and other directions, can solve the problems of high operation intensity, large equipment investment, low reaction yield, etc., and achieves simple operation and equipment investment. The effect of less and higher reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
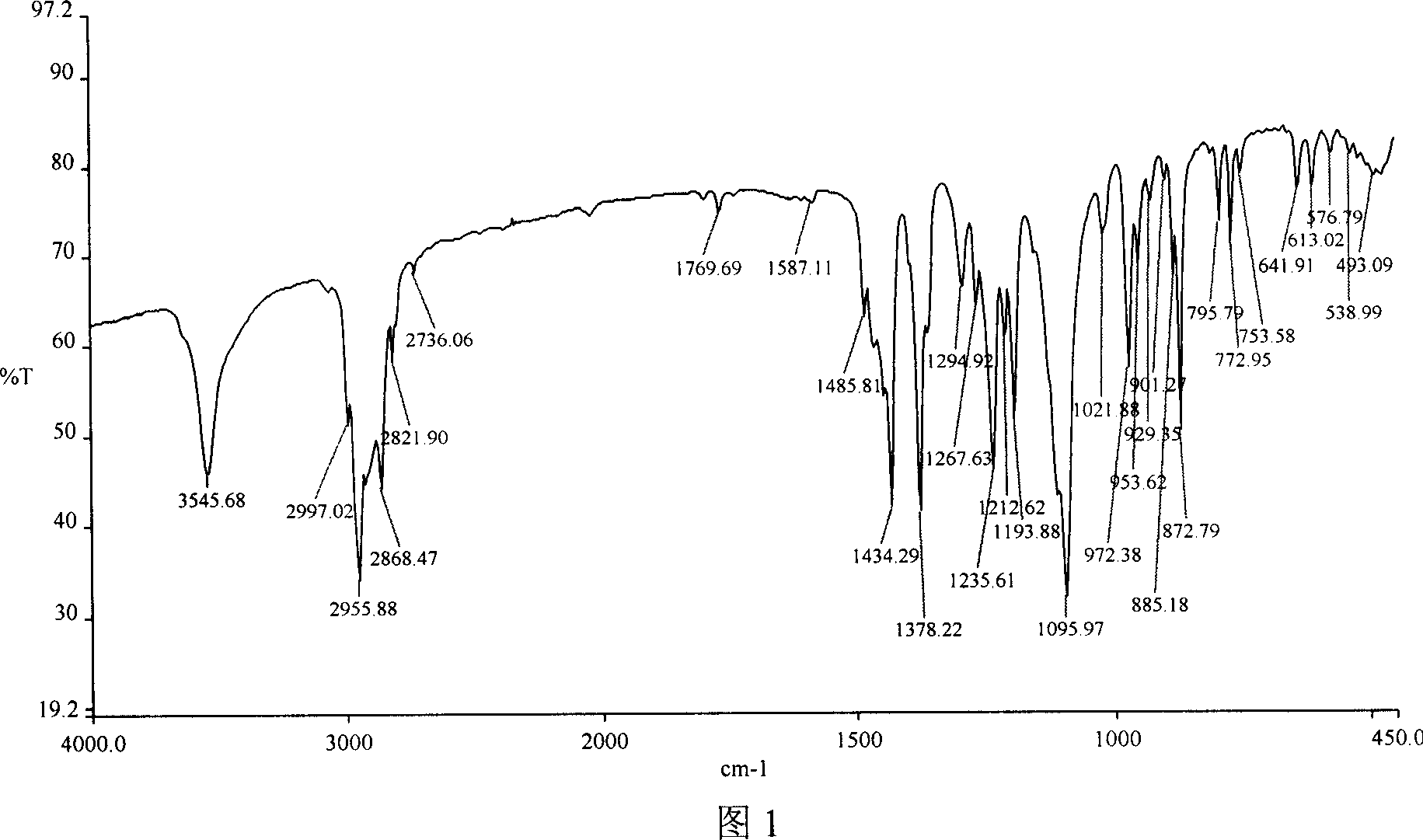
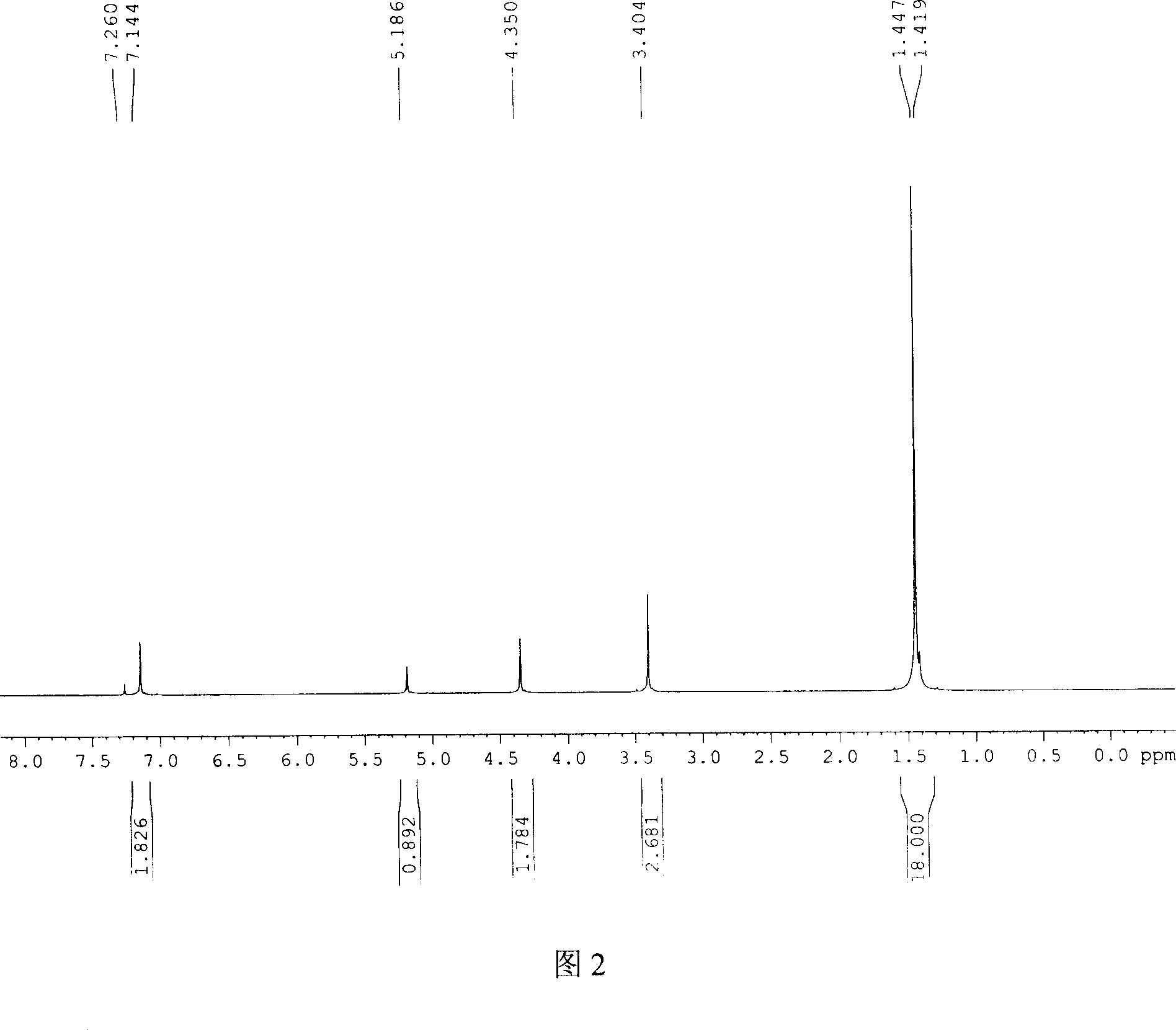
[0059] Example 1 Synthesis of 2,6-di-tert-butyl-4-methoxymethylphenol
[0060] Add 103.0 grams of 2,6-di-tert-butylphenol, 20.0 grams of polyoxymethylene, 360 ml of methanol, 2.0 grams of dimethylamine, and 2.0 grams of triethylamine into a 1000ml round-bottomed flask, reflux at normal pressure, react for 6-8 hours, cool and crystallize 110.0 g of 2,6-di-tert-butyl-4-methoxymethylphenol was obtained with a yield of 88.0% and a melting point of mp98-100°C.
Embodiment 2
[0061] Example 2 Synthesis of 2,6-di-tert-butyl-4-methoxymethylphenol
[0062] Add 103.0 g of 2,6-di-tert-butylphenol, 20.0 g of polyoxymethylene, 360 ml of methanol, 2.0 g of hexahydropyridine, and 2.0 g of triethylamine into a 1000 ml round bottom flask, reflux at normal pressure, react for 6-8 hours, cool and crystallize 110.6 g of 2,6-di-tert-butyl-4-methoxymethylphenol was obtained with a yield of 88.5% and a melting point of mp98-100°C.
Embodiment 3
[0063] Example 3 Synthesis of 2,6-di-tert-butyl-4-methoxymethylphenol
[0064] Add 103.0 grams of 2,6-di-tert-butylphenol, 20.0 grams of polyoxymethylene, 360 ml of methanol, 2.0 grams of dimethylamine, and 2.0 grams of triethylamine into a 1000ml round-bottomed flask, reflux at normal pressure, react for 6-8 hours, and distill off Methanol and other volatile components yielded 124.8 grams of crude product 2,6-di-tert-butyl-4-methoxymethylphenol, with a content of 92.7%.
[0065] Referring to Example 12, 6-di-tert-butyl-4-methoxymethylphenol is synthesized
[0066] Add 103.0 grams of 2,6-di-tert-butylphenol, 20.0 grams of polyoxymethylene, 360 ml of methanol, and 4.0 grams of triethylamine in a 1000 ml round-bottomed flask, reflux at normal pressure, react for 6-8 hours, and no 2,6-di-tert-butylphenol can be obtained. tert-Butyl-4-methoxymethylphenol.
[0067] Referring to Example 2 2,6-di-tert-butyl-4-methoxymethylphenol is synthesized
[0068] Add 103.0 grams of 2,6-di-te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com