Infantile heat-clearing and antitussive dispersing tablets
A dispersible tablet, children's technology, applied in the direction of aluminum/calcium/magnesium active ingredients, medical preparations containing active ingredients, plant/algae/fungus/moss ingredients, etc., can solve adhesion, difficult industrialization, and collapse of traditional Chinese medicine dispersible tablets Solving problems such as difficulty in controlling the speed, achieving the effect of improving the disintegration rate and having a large room for maneuver
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The traditional Chinese medicine raw materials of Xiaoer Qingre Zhike Dispersible Tablets: 90 grams of ephedra, 120 grams of bitter almonds, 270 grams of gypsum, 90 grams of licorice, 180 grams of skullcap, 180 grams of Radix Radix, and 90 grams of Bean Root.
[0054] The weight percentage of traditional Chinese medicine extract and auxiliary materials of Xiaoer Qingre Zhike Dispersible Tablets: 27% of fine powder of Xiaoer Qingre Zhike extract, 20% of pregelatinized starch, 30% of microcrystalline cellulose, 7% of low-substituted hydroxypropyl cellulose, Lactose 10%, polyvinylpyrrolidone 4%, micronized silica gel 1%, magnesium stearate 1%.
[0055] Xiaoer Qingre Zhike Dispersible Tablets are obtained according to the following specific steps: add ephedra and gypsum with 10 times the amount of water and boil for half an hour, then add five flavors such as bitter almonds, then add 8 times the amount of water and boil twice, 2 hours each time, combine The decoction is fil...
Embodiment 2
[0058] 35% by weight of fine powder of Xiaoer Qingrezhike extract, 21% sodium carboxymethylcellulose, 15% cross-linked polyvinylpyrrolidone, 10% microcrystalline cellulose, 5% polyvinylpyrrolidone, 7% lactose, polyethylene Pyrrolidone 4%, micronized silica gel 1%, magnesium lauryl sulfate 2%. All the other are with embodiment 1.
Embodiment 3
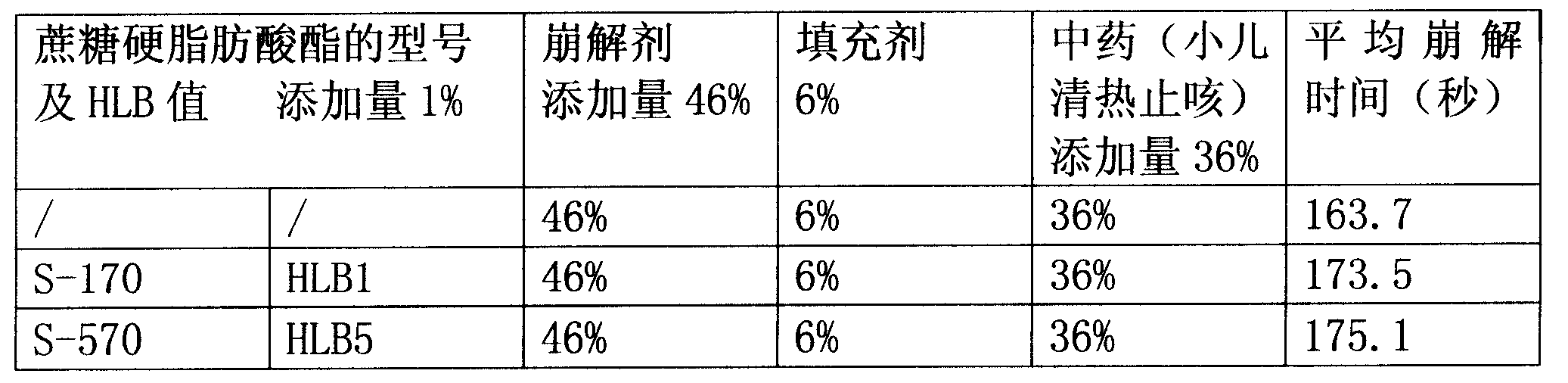
[0060] 40% by weight of fine powder of Xiaoer Qingre Zhike extract, 21% sodium carboxymethyl starch, 15% croscarmellose sodium, 4% low-substituted hydroxypropyl cellulose, 3% cross-linked polyvinylpyrrolidone , mannitol 6%, polyvinylpyrrolidone 7%, micronized silica gel 2%, magnesium stearate 1%, HLB=15-16 sucrose fatty acid ester 1%. Sucrose fatty acid ester is P-1570 or P-1670 or LWA-1570, all the other are the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com